
Culture of Human Cardiomyocytes and its Applications in Drug Discovery and Research

Summary
Human primary cardiomyocyte-based models are superior to whether artificial cellular models or animal models due to their native physiological and pharmacological properties. Therefore, human primary cardiomyocytes have the potential to become the most clinically relevant model for developing novel cardiac drugs and predicting safety assessment of new drugs.- Author Name: AcceGen R&D Team
What are Human Cardiomyocytes?
The heart is one of the first organs formed in the embryo, which highlights its crucial function in pumping blood to carry oxygen and nutrients to other tissues of the organism whether during development or in later life. Human primordial heart starts to beat at around 22 days after fertilization. It is the presence of cardiomyocytes that results in the early beating of the heart. Cardiomyocytes, or cardiac muscle cells are the main type of heart cells and act as the motor unit to drive heart contraction and relaxation. Particularly, adult human primary cardiomyocyte is a preferred in vitro model to evaluate drug-induced cardiotoxicity risk and develop new drugs for cardiac diseases [1].
How to isolate and culture Human Cardiomyocytes?
- 1.Incubate cardiac tissue for 15 min in Krebs-Ringer solution supplemented with protease XXIV.
- 2.Transfer the partially digested tissue to Krebs-Ringer saline containing a combination of collagenase A and hyaluronidase and incubate for 20 min at 37°C with gentle agitation.
- 3.Incubate the tissue with 1.0 mg/ml collagenase A solution, three times for 20 min each at 37°C. Rod-shaped cardiac cells are visible by phase contrast light microscopy after the second incubation step. After each incubation step, transfer the supernatants to a tube, centrifuge at 600 rpm for 4 min, and resuspend the pellets in 1 ml of Ca2+ free Krebs-Ringer solution. Calcium was reintroduced to the pooled suspension by incremental additions of sterile CaCl2 solution (1.0 mol/l) at 4-min intervals until the final concentration of 1.79 mmol/l is achieved.
- 4.Decant the cell suspension into a 10-ml tube, centrifuge at 600 rpm for 4 min and resuspend the pellets with medium 199 (M199) supplemented with 20 mmol/l taurine, 2.0 mmol/l L-carnitine, 5.0 mmol/l creatine, 2.0 mg/ml BSA, 100 μU/ml penicillin and 100 μg/ml streptomycin. Culture medium was further supplemented with either 2% heat inactivated fetal bovine serum (FBS) or with 10−7 mmol/l insulin. Viability of calcium tolerant cells was assessed immediately after isolation by the appearance of a rod-shaped morphology and the exclusion of trypan blue [3].
There are two general types of method for primary cardiomyocytes culture, that is, the dedifferentiated method and the rapid attachment method. Rapid attachment is more routinely used for primary cardiomyocytes cell culture since it leads to better retention of in vivo myocyte morphology and functionality. The rapid attachment method can promote rapid cell attachment using a serum-free medium and coating of the culture substrate with a material, such as laminin, to aid adherence. A major advantage of culture without serum is to prevent proliferation of cardiac fibroblasts and other non-myocyte cells, improving the cellular homogeneity during culture. Cardiomyocytes cultured by rapid attachment are generally quiescent and remain viable within a week of isolation [4]. By the way, human cardiomyocytes are also commercially available. Unlike above mentioned acutely isolated myocytes, the commercially available cardiomyocytes are suitable for longer-term experiments, as they are prepared according to a special protocol.
Human Cardiomyocytes Characteristics
Human Cardiomyocytes morphology can be assessed with phase contrast light microscopy. As shown in Figure 1, isolated atrial and ventricular myocytes are rod-shaped and have sharply defined edges and distinctive sarcomeric bands. Besides, the ultrastructural morphology of cardiomyocytes can be evaluated with transmission electron microscope. Transmission electron micrograph can reveal well-ordered myofilaments with large numbers of glycogen granules, mitochondria, sarcoplasmic reticulum and transverse tubules [2].
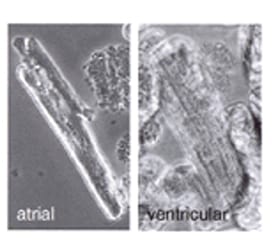
Figure 1 Typical phase contrast micrographs of atrial and ventricular cardiomyocytes isolated from fresh human biopsies (magnification 40×) [3].
Cardiomyocytes function, such as excitation-contraction coupling, is often intimately linked with its shape and morphology. Thus, monitoring these properties may provide indications of cell physiological changes [2].
In cardiomyocytes, there exists a highly developed and complex subcellular machinery which underpins their ability of contraction/relaxation in response to electrical stimulation or exogenously-induced depolarizations. Each cell exhibits a bundle of myofibrils divided into contractile units, or sarcomeres, which consist of contractile proteins and a high density of mitochondria. Most of the cell’s supply of ATP is produced in mitochondria, which render these cells highly resistant to fatigue.
In cardiomyocytes, Ca2+ is the central element of excitation-contraction coupling. And cardiac mechanical contraction is triggered by electrical activation via this intracellular calcium-dependent process. Figure 2 shows Ca2+transport in ventricular myocytes and cardiac excitation-contraction coupling. Dysregulation of intracellular calcium handling in cardiomyocyte is a common feature of heart failure.
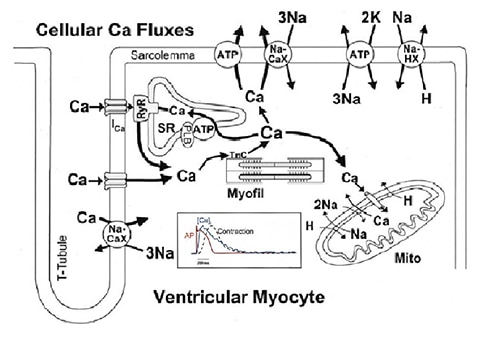
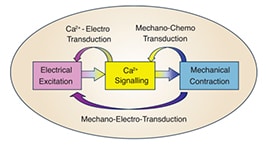
Figure 2. Ca2+transport in ventricular myocytes (upper) and cardiac excitation-contraction coupling (lower) [5, 6].
Applications in Drug Discovery and Research
Drug-induced Cardiotoxicity Risk Prediction
The essential cardiac functions, such as regular heartbeat and myocardial contractility, depend on the electro-mechanical dynamics of cardiac muscle cells. Drug-induced irregular heart beat can limit potential novel therapeutic applications. Therefore, for drug discovery, it is highly recommended to assess the potential of novel drugs to induce pro-arrhythmia and inotropic risk by in vitro cell model. Nathalie Nguyen et al. developed a human cardiomyocyte-based model to measure contractility transients. They deduce both inotropic effect and pro-arrhythmia risk of torsadogenic and non-torsadogenic drugs by using measures of changes in contractility parameters as markers. Their results showed that isolated adult human primary cardiomyocytes can assess human cardiotoxicity and predict inotropic activity and pro-arrhythmia risk during early phases of the drug discovery process [7].
Heart Disease-related Drug Discovery
– Atrial Fibrillation
Atrial fibrillation is the most common cardiac arrhythmia, which leads to an irregular and often abnormally fast heart rate. Most drug discovery for atrial fibrillation concentrate on modulating the ion channels that generally associate with the human atrial myocyteaction potential. Human atrial myocytes have been extensively used to evaluate the effects of atrial ion channel targets. For example, lack of TASK-1 expression in human ventricles might raise the possibility to prolong atrial refractory period without causing ventricular side effects. Thus, blocking atrial TASK-1 channels in atrial cardiomyocytes might beneficially treat or prevent atrial fibrillation [8].
– Heart failure
Heart failure is a major public health problem worldwide. This complex disease results in the heart’s inability to pump enough blood to support other organs in the body. In cardiomyocytes, disrupted Ca2+ homeostasis can lead to the decline in heart pumping function and arrhythmias, which is linked to mortality in heart failure patients [9]. Thus, to correct impaired Ca2+ homeostasis in heart failure, patients is expected to improve contraction/relaxation in cardiomyocytes or arrhythmia suppression. Preparations of normal and failing adult human primary cardiomyocytes have played an important role in supporting the development of therapeutic interventions with direct actions to normalize cardiomyocyte Ca2+ handling [1].
Conclusions
Human primary cardiomyocyte-based models are superior to whether artificial cellular models or animal models due to their native physiological and pharmacological properties. Therefore, human primary cardiomyocytes have the potential to become the most clinically relevant model for developing novel cardiac drugs and predicting safety assessment of new drugs.
What can AcceGen do for you?
Human cardiomyocyte is an excellent in vitro model to evaluate drug-induced cardiotoxicity risk and develop new drugs for cardiac diseases. AcceGen is committed to offering the most authentic Human Cardiomyocytes to help relative research to move forward. AcceGen Human Cardiomyocytes are suitable for long-term experiments, as they are prepared according to a special protocol. For more detailed information, please visit please call us at 1-862-245-6971 or send an email to inquiry@accegen.com. Your questions and requests will be answered by expert staff in AcceGen within 24 hours.
Reference
1. Najah Abi-Gerges, Paul E. Millerand Andre Ghetti: Human Heart Cardiomyocytes in Drug Discovery and Research: NewOpportunities in Translational Sciences. Current Pharmaceutical Biotechnology,2020(21): 787-806.
2. John S Mitcheson, Jules C Hancox, Allan J Levi: Cultured adult cardiac myocytes: Future applications, culture methods, morphological and electrophysiological properties. Cardiovascular Research1998(39): 280–300.
3. S.D. Bird, P.A. Doevendans, M.A. van Rooijen, et al.: The human adult cardiomyocyte phenotype.Cardiovascular Research 2003(58): 423–434.
4. William E. Louch, Katherine A. Sheehanand Beata M. Wolska: Methods in Cardiomyocyte Isolation, Culture, and Gene Transfer. J Mol Cell Cardiol. 2011 (3): 288–298.
5. Bers, D.M.:Cardiac excitation-contraction coupling. Nature, 2002(6868):198-205.
6. Leighton T Izu, Peter Kohl, Penelope A Boyden, et al.: Mechano-Electric and Mechano-Chemo-Transduction in Cardiomyocytes.J Physiol2020(7):1285-1305.
7. Nguyen, N.; Nguyen, W.; Nguyenton, B. et al.: Adult human primary cardiomyocyte-based model for the simultaneous prediction of druginduced inotropic and pro-arrhythmia risk. Front. Physiol., 2017(8): 1073.
8. LimbergS.H., NetterM.F., RolfesC, et al.: TASK-1 channels may modulate action potential duration of human atrial cardiomyocytes. Cell. Physiol. Biochem.,2011(4), 613-624.
9. Peana, D.; Domeier, T.L. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr. Opin. Pharmacol., 2017 (33):17-26.
