
BD Completes Clinical Trial for BD Libertas™ Wearable Injector
GOTHENBURG, Sweden, February 11, 2020 / B3C newswire / -- BD (Becton, Dickinson and Company), a leading global medical technology company, today announced the completion of a 50-subject human clinical trial with the BD Libertas™ Wearable Injector.
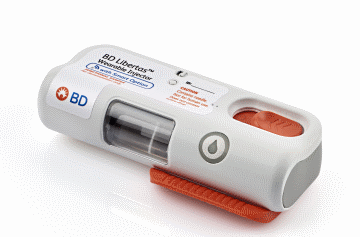
The award-winning injector* is a subcutaneous drug delivery system, currently in development, that is designed to require no patient assembly and deliver biologics with viscosities up to 50 cP in 2-5 mL and 5-10 mL configurations.
The BD independently sponsored and conducted study was designed to evaluate the performance of the 5 mL BD Libertas device in human subjects, including tissue effects, skin reactivity and patient acceptance. The results are expected to be announced early 2020.
The study represents the most recent in a series of over 50 BD conducted pre-clinical and clinical studies intended to measure the performance of the BD Libertas™ Wearable Injector, demonstrate feasibility of 2-10 mL biologic injections into subcutaneous tissue and characterize tissue response to large volume injections in human and animal subjects.
Commenting on the study, Peter Nolan, Worldwide President, BD Pharmaceutical Systems, said, “BD is committed to bringing value to our pharma partnerships, including providing them with independent BD sponsored and generated study data to accelerate combination product development. The recent study reflects BD’s continued investment in solutions to meet pharma’s needs by expanding the design space for biologics delivery.”
For high resolution please click the image.
Editor’s notes
* BD Libertas™ Wearable Injector and DCA Design International – a world leading product design company – have won two of the world’s most prestigious design awards: the Good Design® Award and the iF Award – affirming the quality of the design. The pre-filled, pre-assembled injector is designed to prioritize safety, convenience and usability, while delivering high value drug treatments in the comfort of an in-home environment.
BD Libertas™ Wearable Injector is a product in development; some statements are forward looking and are subject to a variety of risks and uncertainties. BD Libertas™ Wearable Injector is a device component intended for drug-device combination products and not subject to FDA 510(k) clearance.
About BD
BD is one of the largest global medical technology companies and is advancing healthcare by improving medical discovery, diagnostics and care delivery. The company supports the heroes that work on the frontline by developing innovative technology, services and solutions, to advance clinical therapy for patients and clinical process for healthcare providers. BD and its 65,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians' care delivery process, enable laboratory scientists to accurately detect disease and advance researchers' capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to healthcare.
Contacts
Troy Kirkpatrick
BD Public Relations, Senior Director
(001) 858.617.2361
troy.kirkpatrick@bd.com
Monique N. Dolecki
BD Investor Relations
(001) 201.847.5378
monique_dolecki@bd.com
Keywords: Humans; Subcutaneous Tissue; Biological Products; Pharmaceutical Preparations; Drug Delivery Systems; Injections; Wearable Electronic Devices
Published by B3C newswire and shared through Newronic®
Editor Details
-
Company:
- B3C newswire
- Website:

