
GeneTex Launches Novel Glycan Array-Validated CA 19-9 Antibody
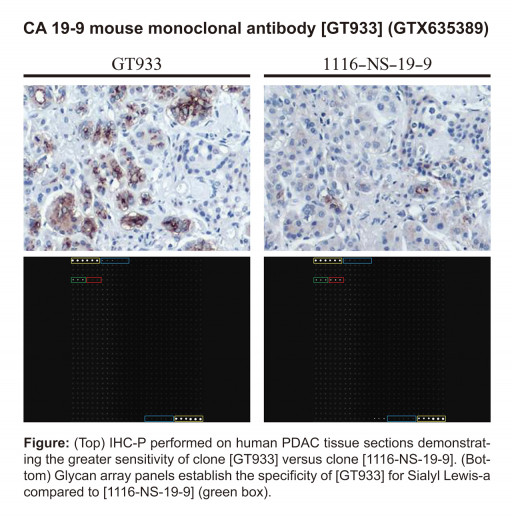
CA19-9 IHC and Glycan Array Data
IHC-P performed on human PDAC tissue sections and glycan array panels
IRVINE, Calif. - April 29, 2021 - (Newswire.com)
GeneTex has developed antibodies against tumor markers for over 20 years. Most recently, the company has focused its efforts on CA 19-9 (also known as Sialyl Lewis-a), a cell surface glycolipid and O-linked glycoprotein complex whose measurement is widely used clinically in the diagnosis, management, prognosis, and early recurrence detection of pancreatic ductal adenocarcinoma (PDAC). Additional research is examining the potential use of CA 19-9-specific antibodies as therapeutics against metastatic PDAC, as well as the value of this biomarker for assessing other benign conditions and malignancies affecting gastrointestinal (GI) and non-GI organ systems.
GeneTex presently offers a panel of four mouse monoclonal antibodies that detect CA 19-9 (GTX635388, GTX635389, GTX635390, GTX635391). All four of these reagents are validated for multiple applications, including immunoblot, immunocytochemistry (ICC/IF), immunohistochemistry (IHC), flow cytometry/FACS, ELISA, and sandwich ELISA. In addition, comparative studies have shown that each of these antibodies has similar or higher sensitivity (for both immunoblot and IHC) than the 1116-NS-19-9 monoclonal antibody that was first used to describe CA 19-9. The supportive information for GTX635389 is particularly compelling, as it illustrates not only a robustly stronger sensitivity for CA 19-9 but also glycan array data demonstrating clear specificity for Sialyl Lewis-a. This is another feature that distinguishes this antibody from 1116-NS-19-9, and suggests that it could therefore be particularly advantageous in diagnostic applications. The thorough and exhaustive validation of these four antibodies, especially in the case of GTX635389, indicates that they can be used individually or in combination to enhance studies of PDAC and other tumors, as well as of a number of non-neoplastic conditions, where CA 19-9 expression is known or suspected.
Tumor markers already play pivotal roles in the diagnosis, treatment decisions, prognosis, and surveillance of multiple cancers. Nevertheless, for most malignancies no reliable biomarker is available, and this represents a major problem in oncology and other clinical fields. GeneTex remains dedicated to providing the reagents necessary for future advances in biomarker discovery and characterization.
GeneTex products are for research use only. Not for diagnostic or therapeutic procedures.
Media Contact:
Allen Lee
Phone: 949.553.1900
Email: allensl@genetex.com
Press Release Service by Newswire.com
Original Source: GeneTex Launches Novel Glycan Array-Validated CA 19-9 Antibody
Editor Details
-
Company:
- Newswire
