
ZAP Surgical Appoints Industry Veterans to Lead Chinese Market Expansion
Chinese Clinical Trials for Next-Gen ZAP-X Gyroscopic Radiosurgery Have Concluded, NMPA Regulatory Clearance Expected Soon
SAN CARLOS, Calif.--(BUSINESS WIRE)--#braintumors--ZAP Surgical Systems, Inc., maker of the ZAP-X® Gyroscopic Radiosurgery® platform, today announced the appointment of Jie Sun, PhD, as Vice President, Greater China, and Calvin Gao as China General Manager. Prior to NMPA (National Medical Products Administration) regulatory clearance, Mr. Sun and Mr. Gao will pave the way for the commercialization and adoption of ZAP-X for novel and non-invasive brain tumor treatments in China.
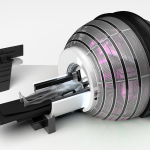

The company also announced that the ZAP-X clinical trial in China has completed. As part of the China NMPA clearance process, the study assessing patient safety and treatment efficacy is currently under review and the ZAP-X looks to soon be approved for broad clinical use in China.
Prior to joining ZAP Surgical, Mr. Sun was the founding president of Edan Medical USA in Sunnyvale, CA. As part of Edan’s leadership team, Mr. Sun contributed to the company’s operational efforts as well as successful US, European, and Chinese market penetration strategies.
Mr. Gao comes to ZAP Surgical from the surgical robotics business of Beijing Baihui Weikang Technology Co., Ltd, where he served as National Marketing and Sales Director.
“We are very excited to have both Jie and Calvin join the ZAP Surgical team,” said Dr. John Adler, CEO of ZAP Surgical Systems, and professor emeritus of neurosurgery and radiation oncology at Stanford University. “Their combined experience in medical devices and expertise in the Chinese marketplace bring incredible value to the organization and will surely contribute to ZAP’s vision for rapid market penetration.”
Radiosurgery, also referred to as stereotactic radiosurgery (SRS), is a well-established and effective treatment for many brain cancers including primary and metastatic brain tumors. Considered an alternative to surgery for select indications, SRS is a non-invasive outpatient procedure that often provides superior outcomes, yet requires no surgical incision, and little to no patient recovery period.
U.S. FDA-cleared in September of 2017, Japanese Shonin-cleared in 2020, and European CE-cleared in 2021, ZAP-X utilizes a modern linear accelerator for radiation beam generation, thereby eliminating the historical use of Cobalt-60 and the related costs and challenges of handling live radioactive isotopes. ZAP-X also incorporates a unique vault-free design that typically eliminates the need for costly shielded treatment rooms.
For additional information about the ZAP-X, please visit: https://zapsurgical.com/. Detailed system overview animations can be found at https://youtu.be/9ph-cdb5QO0.
About ZAP Surgical Systems, Inc.
ZAP Surgical Systems, Inc. designs and manufactures the ZAP-X® Gyroscopic Radiosurgery® platform. ZAP was founded in 2014 by Dr. John R. Adler, CEO and founder of ZAP Surgical, and Emeritus Dorothy & TK Chan Professor of Neurosurgery and Radiation Oncology at Stanford University. The ZAP-X platform incorporates a unique vault-free design that typically eliminates the need for costly shielded treatment rooms. ZAP-X also utilizes a modern linear accelerator to eliminate historical use of Cobalt-60. Learn more at www.zapsurgical.com, or follow us on LinkedIn (https://www.linkedin.com/company/zapsurgical/) and Twitter (https://twitter.com/ZapSurgical).
Contacts
Mark Arnold
ZAP Surgical Systems, Inc.
Senior Vice President, Marketing
+1 650 492 7797, ext. 101
Email: info@zapsurgical.com
Editor Details
-
Company:
- Businesswire
