
Arcturus Therapeutics Announces Third Quarter 2022 Financial Update and Pipeline Progress
Entered into global partnership with CSL to develop and commercialize self-amplifying mRNA vaccines targeting COVID-19, influenza, additional pathogens, and pandemic preparedness with $200 million upfront and up to $4.3 billion in potential development and commercial milestones
BARDA award announced for up to $63.2 million to support development of a self-amplifying mRNA vaccine for rapid pandemic influenza response
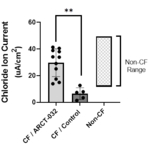
New LUNAR-CF preclinical data demonstrates effective delivery of ARCT-032 with LUNAR®, resulting in functional restoration of chloride ion current in CF subject bronchial epithelial cells
Investor conference call at 4:30 p.m. EST today
SAN DIEGO--(BUSINESS WIRE)--Arcturus Therapeutics Holdings Inc. (the “Company”, “Arcturus”, “Arcturus Therapeutics”, Nasdaq: ARCT), a global late-stage clinical messenger RNA medicines company focused on the development of infectious disease vaccines and significant opportunities within liver and respiratory rare diseases, today announced its financial results for the third quarter ended September 30, 2022, and provided corporate updates.

"Arcturus continues to execute on the promise of our next-generation self-amplifying mRNA vaccine franchise through engagements with CSL and BARDA," said Joseph Payne, President and CEO of Arcturus Therapeutics. "These new partnerships expand the scope of our technology while providing substantial capital to accelerate the development of our vaccine and therapeutic programs. In addition, promising new data from ARCT-032, our CF therapeutic candidate, was recently featured at the North American Cystic Fibrosis Conference and we expect to file a CTA before year end."
Recent Corporate Highlights
- Announced a strategic collaboration with CSL Seqirus, one of the top two global influenza vaccine companies, for the development, manufacture, and commercialization of mRNA-based vaccines. Arcturus will receive $200 million upfront, up to $4.3 billion in potential development and commercial milestones, 40% profit sharing for COVID-19 vaccines and up to double digit royalties for influenza and three additional respiratory infectious disease vaccines. The collaboration combines CSL Seqirus’ established global vaccine commercial and manufacturing infrastructure with Arcturus’ expertise in mRNA design and modification, LUNAR® lipid nanoparticle (LNP) technology and manufacturing know-how. Previously reported clinical results from ongoing ARCT-154 studies have demonstrated a favorable efficacy and safety profile with sustained neutralizing antibodies against COVID-19, including recent variants of concern.
- The Biomedical Advanced Research and Development Authority (BARDA) provided Arcturus with an award valued at up to $63.2 million over three years to support preclinical, manufacturing, nonclinical safety studies, along with development and regulatory support for Arcturus’ self-amplifying mRNA vaccine platform technology for rapid pandemic influenza response through Phase 1 clinical studies.
- ARCT-810, the Company’s mRNA therapeutic candidate for OTC deficiency is being evaluated in a randomized, double-blind, placebo-controlled, nested single and multiple ascending dose Phase 2 study in 24 adolescents and adults with OTC deficiency. Participating sites have identified several dozen patients in pre-screening with dosing to begin Q4 2022. All subjects in the Phase 1b single ascending dose (SAD) study have completed dosing, including the cohort dosed at 0.4 mg/kg, without requiring steroid co-treatment. The Company will share interim ARCT-810 clinical data when we announce additional liver therapeutic programs in 2023.
- New preclinical data from ARCT-032, the Company’s inhaled mRNA therapeutic for cystic fibrosis, demonstrated effective delivery of mRNA to bronchial and tracheal epithelial cells in the presence of CF sputum utilizing Arcturus’ proprietary LUNAR® technology (CF Ferret G551D model). Additional in vitro data demonstrated robust restoration of CFTR activity. Bronchial epithelial cells (BECs) obtained from human CF donors were treated with ARCT-032 and exhibited a significant increase in chloride ion current compared to control BECs obtained from non-CF donors (see Figure). ARCT-032 remains on track for CTA filing by year end 2022.
Financial Results for Third Quarter Ended September 30, 2022
Revenues in conjunction with strategic alliances and collaborations: Arcturus’ primary sources of revenues were from consulting and related technology transfer fees, reservation fees, license fees and collaborative payments received from research and development arrangements with pharmaceutical and biotechnology partners. For the three months ended September 30, 2022, the Company reported revenue of $13.4 million compared with $2.4 million for the three months ended September 30, 2021, and $27.1 million for the three months ended June 30, 2022. Revenue increased by $10.9 million during the three months ended September 30, 2022, as compared to the three months ended September 30, 2021, which primarily relates to an increase in revenue related to the agreement with Vinbiocare.
Operating expenses: Total operating expenses for the three months ended September 30, 2022, were $50.2 million compared with $56.3 million for the three months ended September 30, 2021, and $49.2 million for the three months ended June 30, 2022. The decline in operating expenses when compared to the three months ended September 30, 2021 was primarily due to lower manufacturing related expenses.
Research and development expenses: Research and development expenses for the three months ended September 30, 2022, were $37.7 million compared with $45.4 million for the three months ended September 30, 2021, and $38.2 million for the three months ended June 30, 2022. The decline in research and development expenses when compared to the three months ended September 30, 2021 was primarily due to lower manufacturing related expenses.
Net Loss: For the three months ended September 30, 2022, Arcturus reported a net loss of approximately $35.3 million, or ($1.33) per basic and diluted share, compared with a net loss of $54.1 million, or ($2.05) per basic and diluted share in the three months ended September 30, 2021, and a net loss of $21.6 million, or ($0.82) per basic and diluted share in the three months ended June 30, 2022.
Cash Position: The Company’s cash balance totaled $237.7 million as of September 30, 2022, compared to a cash balance of $370.5 million at December 31, 2021.
Earnings Call: Wednesday, November 9, 2022 @ 4:30 pm EST
Domestic: 1-888-204-4368
International: 1-323-994-2093
Conference ID: 2581187
Webcast: Link
About Arcturus Therapeutics
Founded in 2013 and based in San Diego, California, Arcturus Therapeutics Holdings Inc. (Nasdaq: ARCT) is a global, late-stage clinical mRNA medicines and vaccines company with enabling technologies: (i) LUNAR® lipid-mediated delivery, (ii) STARR™ mRNA Technology (samRNA) and (iii) mRNA drug substance along with drug product manufacturing expertise. Arcturus’ diverse pipeline of RNA therapeutic and vaccine candidates includes mRNA vaccine programs for SARS-CoV-2 (COVID-19) and Influenza, and other programs to potentially treat ornithine transcarbamylase (OTC) deficiency, and cystic fibrosis, along with partnered programs including glycogen storage disease type III, and hepatitis B virus. Arcturus’ versatile RNA therapeutics platforms can be applied toward multiple types of nucleic acid medicines including messenger RNA, small interfering RNA, circular RNA, antisense RNA, self-amplifying RNA, DNA, and gene editing therapeutics. Arcturus’ technologies are covered by its extensive patent portfolio (patents and patent applications issued in the U.S., Europe, Japan, China and other countries). For more information, visit www.ArcturusRx.com. In addition, please connect with us on Twitter and LinkedIn.
Forward Looking Statements
This press release contains forward-looking statements that involve substantial risks and uncertainties for purposes of the safe harbor provided by the Private Securities Litigation Reform Act of 1995. Any statements, other than statements of historical fact included in this press release, are forward-looking statements, including those regarding strategy, future operations, the likelihood of success of the Company’s pipeline (including ARCT-032, ARCT-810 and ARCT-154) the expectations for beginning the collaboration with CSL Seqirus, including receiving clearance under the Hart-Scott-Rodino Antitrust Improvements Act and satisfying other closing conditions, or the likelihood of success of the collaboration with CSL Seqirus or any collaborations including the achievement of any milestones or other payments, the future activities under and fulfillment of the Company’s contract with BARDA, the ability of the Company’s influenza vaccine program to support U.S. government pandemic preparedness goals, the likelihood that preclinical (including for ARCT-032 therapeutic candidate) or clinical data will be predictive of future clinical results, the anticipated timing for filing of a CTA for ARCT-032, the ability of the Company to initiate, enroll and execute clinical trials (including the ARCT-810 trial), the timing for sharing interim ARCT-810 clinical data or announcing additional liver therapeutic programs, the likelihood that results to date for ARCT-154 or any other clinical candidate will be predictive of future clinical results, including with respect to future variants of concern or sufficient for regulatory approval, the timing and nature of any study results, the potential administration regimen or dosage, or ability to administer multiple doses of, any of the Company’s drug candidates, the likelihood that a patent will issue from any patent application, its current cash position and expected cash burn and the impact of general business and economic conditions. Arcturus may not actually achieve the plans, carry out the intentions or meet the expectations or projections disclosed in any forward-looking statements such as the foregoing and you should not place undue reliance on such forward-looking statements. These statements are only current predictions or expectations, and are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward-looking statements, including those discussed under the heading "Risk Factors" in Arcturus’ most recent Annual Report on Form 10-K, and in subsequent filings with, or submissions to, the SEC, which are available on the SEC’s website at www.sec.gov. Except as otherwise required by law, Arcturus disclaims any intention or obligation to update or revise any forward-looking statements, which speak only as of the date they were made, whether as a result of new information, future events or circumstances or otherwise.
Trademark Acknowledgements
The Arcturus logo and other trademarks of Arcturus appearing in this announcement, including LUNAR® and STARR™, are the property of Arcturus. All other trademarks, services marks and trade names in this announcement are the property of their respective owners.
ARCTURUS THERAPEUTICS HOLDINGS INC. AND ITS SUBSIDIARIES CONDENSED CONSOLIDATED BALANCE SHEETS |
||||||||
|
|
September 30,
|
|
|
December 31,
|
|
||
(in thousands, except par value information) |
|
(unaudited) |
|
|
|
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
237,676 |
|
|
$ |
370,492 |
|
Accounts receivable |
|
|
2,044 |
|
|
|
3,367 |
|
Prepaid expenses and other current assets |
|
|
6,960 |
|
|
|
5,102 |
|
Total current assets |
|
|
246,680 |
|
|
|
378,961 |
|
Property and equipment, net |
|
|
11,347 |
|
|
|
5,643 |
|
Operating lease right-of-use asset, net |
|
|
33,519 |
|
|
|
5,618 |
|
Equity-method investment |
|
|
— |
|
|
|
515 |
|
Non-current restricted cash |
|
|
2,081 |
|
|
|
2,077 |
|
Total assets |
|
$ |
293,627 |
|
|
$ |
392,814 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
17,962 |
|
|
$ |
10,058 |
|
Accrued liabilities |
|
|
25,529 |
|
|
|
23,523 |
|
Current portion of long-term debt |
|
|
27,702 |
|
|
|
22,474 |
|
Deferred revenue |
|
|
4,656 |
|
|
|
43,482 |
|
Total current liabilities |
|
|
75,849 |
|
|
|
99,537 |
|
Deferred revenue, net of current portion |
|
|
5,179 |
|
|
|
19,931 |
|
Long-term debt, net of current portion |
|
|
32,038 |
|
|
|
40,633 |
|
Operating lease liability, net of current portion |
|
|
31,218 |
|
|
|
4,502 |
|
Other non-current liabilities |
|
|
3,676 |
|
|
|
— |
|
Total liabilities |
|
$ |
147,960 |
|
|
$ |
164,603 |
|
Stockholders’ equity |
|
|
|
|
|
|
||
Common stock, $0.001 par value; 60,000 shares authorized; issued and outstanding shares were 26,492 at September 30, 2022 and 26,372 at December 31, 2021 |
|
|
26 |
|
|
|
26 |
|
Additional paid-in capital |
|
|
601,129 |
|
|
|
575,675 |
|
Accumulated deficit |
|
|
(455,488 |
) |
|
|
(347,490 |
) |
Total stockholders’ equity |
|
|
145,667 |
|
|
|
228,211 |
|
Total liabilities and stockholders’ equity |
|
$ |
293,627 |
|
|
$ |
392,814 |
|
ARCTURUS THERAPEUTICS HOLDINGS INC. AND ITS SUBSIDIARIES CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE LOSS (unaudited) |
||||||||||||
|
|
Three Months Ended |
|
|||||||||
|
|
September 30, |
|
|
June 30, |
|
||||||
(in thousands, except per share data) |
|
2022 |
|
|
2021 |
|
|
2022 |
|
|||
Revenue |
|
$ |
13,369 |
|
|
$ |
2,437 |
|
|
$ |
27,093 |
|
Operating expenses: |
|
|
|
|
|
|
|
|
|
|||
Research and development, net |
|
|
37,688 |
|
|
|
45,398 |
|
|
|
38,189 |
|
General and administrative |
|
|
12,488 |
|
|
|
10,860 |
|
|
|
10,993 |
|
Total operating expenses |
|
|
50,176 |
|
|
|
56,258 |
|
|
|
49,182 |
|
Loss from operations |
|
|
(36,807 |
) |
|
|
(53,821 |
) |
|
|
(22,089 |
) |
Loss from equity-method investment |
|
|
— |
|
|
|
(250 |
) |
|
|
(131 |
) |
Gain from foreign currency |
|
|
1,862 |
|
|
|
506 |
|
|
|
1,217 |
|
Finance expense, net |
|
|
(321 |
) |
|
|
(519 |
) |
|
|
(560 |
) |
Net loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
Net loss per share, basic and diluted |
|
$ |
(1.33 |
) |
|
$ |
(2.05 |
) |
|
$ |
(0.82 |
) |
Weighted-average shares outstanding, basic and diluted |
|
|
26,467 |
|
|
|
26,338 |
|
|
|
26,425 |
|
Comprehensive loss: |
|
|
|
|
|
|
|
|
|
|||
Net loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
Comprehensive loss |
|
$ |
(35,266 |
) |
|
$ |
(54,084 |
) |
|
$ |
(21,563 |
) |
Contacts
IR and Media Contacts
Arcturus Therapeutics
IR@arcturusrx.com
Kendall Investor Relations
Carlo Tanzi, Ph.D.
(617) 914-0008
ctanzi@kendallir.com
Editor Details
-
Company:
- Businesswire
