
GeneTex Addresses GPCR Targets With Its Recombinant Monoclonal Antibody Production Platform
GPCR Validation Data
IRVINE, Calif., August 14, 2023 (Newswire.com) - GeneTex, a multinational antibody manufacturing company, is leveraging its recombinant monoclonal antibody production platform and augmented validation protocols to generate immunological reagents against high-value targets that have historically proven difficult to produce. G protein-coupled receptors (GPCRs), one of the most notoriously challenging groups, constitute the largest membrane protein superfamily in the human genome with more than 800 genes. These multipass membrane proteins are involved in a myriad of essential functions in normal physiology and in many diseases. Importantly, almost a third of therapeutics in use today are directed against GPCRs, and future drug development will benefit from well-validated antibodies against other GPCRs. Thus, the need for dependable antibodies to study these proteins among scientists in the academic, clinical, and pharmaceutical fields is critical.
As noted above, the development of antibodies specific for individual GPCRs has proven to be technically difficult. Much of the GPCR structure is buried in the cell membrane. In addition, cell expression levels are often quite low for many GPCRs, and identifying an immunoreactive antigen that recapitulates the required conformational structure while also being specific for a particular GPCR can be elusive. Antibody specificity for the targeted GPCR and unambiguous demonstration of performance in experimental applications (e.g., western blot, immunohistochemistry, and immunocytochemistry) valued by researchers are equally formidable to establish.
GeneTex is relying on the advantages of its recombinant antibody technology as well as enhanced validation strategies to surmount these obstacles. For production, the recombinant antibody workflow allows the detection of promising clones early in the process and scalability and consistency after the clones are selected. GeneTex's validation efforts will focus on CRISPR-based knockout (KO) protocols coupled with the implementation of VirDTM-GPCR arrays (developed by CDI Labs, Baltimore, MD), which present a nearly comprehensive library of human non-olfactory GPCRs individually expressed on virion envelopes. These arrays allow the most effective large-scale screening mechanism to test the specificity of any human GPCR-binding reagent. Scott Paschke, Vice President at CDI Labs, states, "We are excited to be working with GeneTex on their efforts to produce highly specific GPCR antibodies. CDI's GPCR VirDTM arrays are produced by a patented method that expresses the human GPCR protein through the use of an engineered HSV-1 virus. This system yields a recombinant human GPCR protein embedded in a human membrane to ensure correct folding. The VirDTM system is a perfect tool for assaying GPCR antibody specificity."
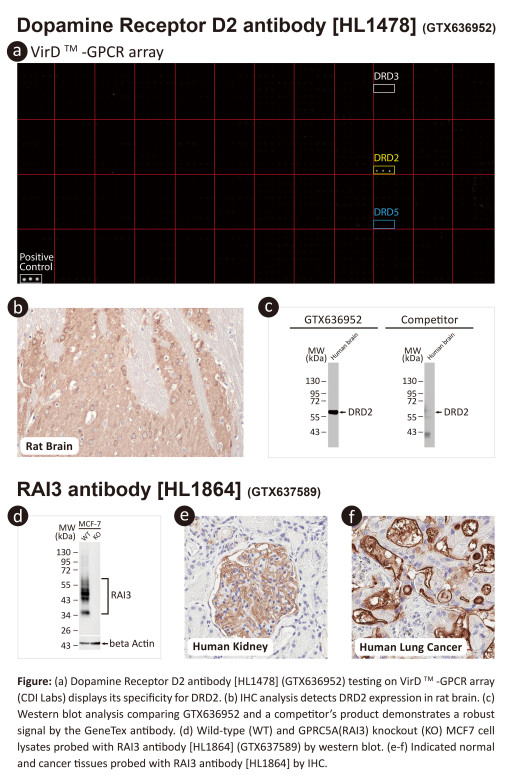
These efforts by GeneTex have already produced new antibodies for two important GPCRs. The first is the new D2 dopamine receptor (DRD2) antibody (GTX636952) that targets one of the five GPCRs responsible for dopamine signaling in humans. DRD2 is particularly significant as the target of drugs used to treat psychoses and Parkinson's disease. GeneTex's recombinant antibody is validated using comparable antibodies, differential tissue expression, and the VirDTM-GPCR array in the process of affirming its use in several applications. The array data was remarkable for a distinct signal for DRD2 but not for DRD3, DRD5, or any other GPCR or membrane protein on the array, which argues that the antibody is specific for DRD2. A second recombinant antibody for retinoic acid-induced protein 3 (RAI3/GPRC5A) (GTX637589), which may be a prognostic marker for several major human malignancies, was validated using CRISPR KO, tissue staining, and differential cell line expression. These two reagents are just the first of what GeneTex hopes will be an extensive and highly refined catalog of GPCR antibodies to meet the needs of GPCR researchers. Allen Lee, GeneTex's Vice President of Business Development, commented, "Over the next two years, GeneTex will produce the most comprehensive portfolio of best-in-class antibodies against GPCRs. We hope this will accelerate drug discovery and research."
Contact Information:Allen Lee
Vice President - Business Development
allensl@genetex.com
(949)553-1900
Original Source: GeneTex Addresses GPCR Targets With Its Recombinant Monoclonal Antibody Production Platform
Editor Details
-
Company:
- Newswire
