
CurvaFix Showcases Novel, Curved Intramedullary Devices for Pelvic Fixation at ACEP Scientific Assembly
CurvaFix® IM Implant delivers robust, stable pelvic fracture fixation even in weakened bones
BELLEVUE, Wash.--(BUSINESS WIRE)--#ACEP2023--CurvaFix, Inc., a developer of medical devices to repair fractures in curved bones, announced today it will showcase the CurvaFix® IM Implants for geriatric fragility fractures of the pelvis (FFP) at the American College of Emergency Physicians (ACEP) Scientific Assembly in Philadelphia, October 9-11, 2023, in booth #1935.
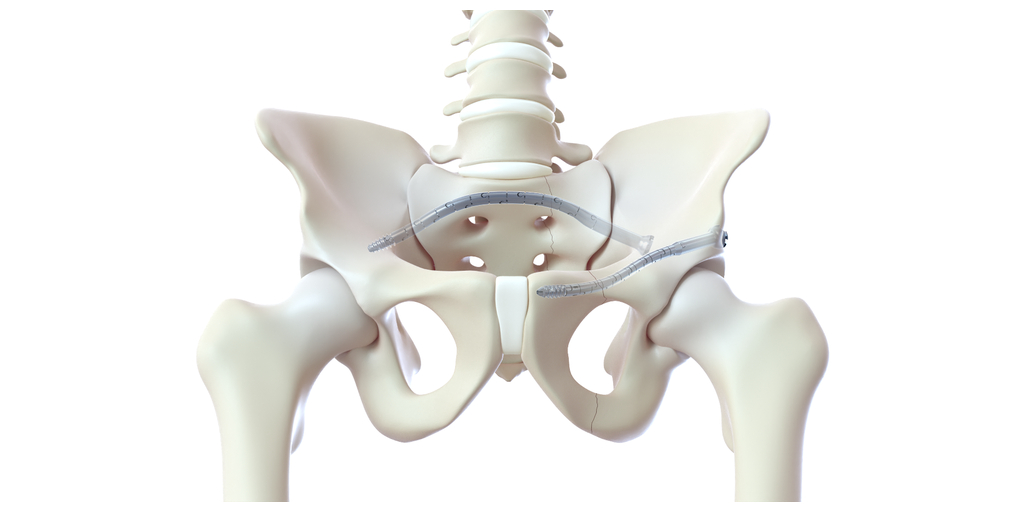


“CurvaFix is focused on restoring mobility and renewing lives. As such, we are pleased to make our debut at the ACEP Scientific Assembly, the world’s largest emergency medicine educational conference,” said Mark Foster, chief executive officer at CurvaFix. “Emergency Medicine physicians are often the first point of care for patients who sustain geriatric pelvic fractures or fragility fractures of the pelvis.
“As the orthopedic trauma community is adopting new technology to help manage these patients better, it’s important to update other physicians within the patient care pathway,” continued Foster. “We have a growing number of orthopedic trauma surgeons across the U.S. who now have access to a surgical alternative that can help patients regain their mobility and their lives with the CurvaFix IM Implant.”
A majority of patients who sustain FFPs receive non-surgical care (conservative care), including bed rest, pain medication and mobility assistance. Often, patients experience a worsening of symptoms leading to lengthy hospitalizations, high nursing home admittance, and a high rate of mortality. Although not every patient who experiences FFP is a candidate or in need of surgery, increasing clinical evidence suggests that more patients could benefit from surgical care than receive it today.
“I’ve been pleasantly surprised with the flexibility of the CurvaFix devices, and I believe they will continue to play a role in pelvic fixation, especially in osteoporotic patients,” said Brett Crist, M.D., Director of Orthopaedic Trauma Service and Orthopaedic Trauma Fellowship at the University of Missouri in Columbia, Mo. “In one of our cases, we treated a 63-year-old morbidly obese female (BMI 46.5) patient who sustained a low energy fall two years prior, resulting in pelvic fragility fractures. Her treatment had been non-operative management, leaving her wheelchair-dependent before eventually being referred to me. Based on her pre-operative CT scan, sacral dysmorphism, and bone quality, I thought the 7.5mm CurvaFix Implant would work well and that the maneuverable ball-tipped guide wire would aid us in being able to align the rami for fixation. Pleased with the stability that the 9.5mm CurvaFix device provides in osteoporotic patients, I wanted to use the smaller device to be able to place it in the smaller corridor.
“Overall, I was very pleased to be able to use the new 7.5mm CurvaFix devices for the left and right rami nonunions and the second sacral corridor while also being very satisfied with the smoothness of the anterior ring fixation,” continued Dr. Crist. “Following two years of being dependent on a wheelchair, this patient was discharged from the hospital with a walker within 24 hours of her CurvaFix procedure. Moreover, her visual analog scale for pain (VAS) was between an 8 and 9 prior to her surgery, and within 12 hours of the procedure, her VAS pain score was down to a 3.”
Since receiving clearance from the U.S. Food & Drug Administration (FDA), CurvaFix Implants have been used to treat hundreds of patients who have sustained fragility fractures of the pelvis, highlighting the benefits of surgically stabilizing FFPs in elderly patients, including the potential for faster reduction of pain and earlier weight-bearing and mobility when compared to conservative care.
About CurvaFix, Inc.
CurvaFix, Inc. is a privately held medical device company headquartered in Bellevue, Wash. The company is developing implantable products to improve fracture repair in curved bones and is focusing on Fragility Fractures of the Pelvis (FFP) and high-impact pelvic fractures. The CurvaFix IM Implant has received 510(k) clearance from the U.S. Food & Drug Administration (FDA).
Contacts
Amy Cook
Acook@curvafix.com
Editor Details
-
Company:
- Businesswire
