
GeneTex Augments Its GPCR Reagent Portfolio With a Highly Specific CXCR2 Recombinant Monoclonal Antibody
CXCR2 Antibody [HL2604] Validation
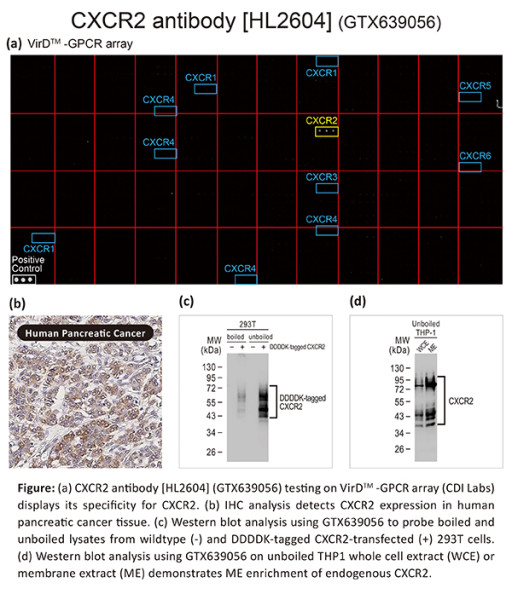
CXCR2 Antibody [HL2604] testing on GPCR array, IHC analysis, and Western blot analysis.
IRVINE, Calif., November 15, 2023 (Newswire.com) - GeneTex, a multinational antibody manufacturer, announces a new addition to its growing product line of validated recombinant monoclonal antibodies against G protein-coupled receptors (GPCRs). CXC motif chemokine receptor 2 (CXCR2) belongs to the family of 20 human chemokine receptors responsible for signal transduction triggered by the binding of one or more of 46 chemokines. Chemokines and their respective receptors, which normally are involved in the immune response, are also active in a plethora of diseases, including inflammatory and autoimmune disorders, neurological disease, and solid and hematologic cancers, among others. Though there is a significant body of literature on chemokines, there are fewer studies on the receptors. Novel reagents that are specific for particular CXC (and other chemokine) receptors are essential to promote additional research.
Human GPCRs are expressed by over 820 genes that code for highly dynamic, seven-transmembrane domain proteins that respond to ligands of various types. GPCRs elicit profound physiological and pathophysiological effects that are relevant to many chronic diseases, including type 2 diabetes mellitus, malignancies, mood disorders, obesity, and neurodegenerative disease. Approximately 350 of the non-olfactory GPCRs are considered amenable to pharmaceutical intervention, though the vast majority remain without available drugs. Research into GPCR biology is of great importance, and GeneTex is committed to producing a comprehensive GPCR antibody portfolio to promote this work.
Due to well-known technical challenges associated with antibody development for specific GPCR targets, GeneTex employs enhanced and/or novel validation modalities to establish specificity among GPCR family members, particularly those that share sequence identity. These strategies include knockout/knockdown testing, immunohistochemistry (IHC) or immunocytochemistry (ICC/IF) staining of tissues/cells with differential target expression, and comparison of signals between antibodies against the same target, among other approaches. In addition, GeneTex is incorporating the innovative VirDTM-GPCR arrays (CDI Labs, Baltimore, MD) to determine specificity. In the case of the new recombinant monoclonal CXCR2 antibody (GTX639056), this array demonstrates absolute specificity for CXCR2, with no cross-reactivity detected for CXCR1, CXCR3, CXCR4, CXCR5, and CXCR6. This is particularly significant as CXCR1 and CXCR2 share the highest similarity with ~77% sequence identity. This array data is complemented by both western blot (WB) and IHC validation. As expected, the WB shows a significantly more robust signal in a “membrane extract” fraction compared to the matching “whole cell extract” sample. For IHC, human pancreatic cancer tissue staining reveals the anticipated signal enrichment in the cytoplasm and at the cell membrane.
GeneTex is committed to providing the most extensive and thoroughly validated recombinant monoclonal antibody repertoire for GPCR proteins. For the chemokine receptors specifically, efforts are currently underway to broaden the catalog of reagents against the remaining CXC, CC, CX3C, and XC receptors. This same large-scale approach will be applied to all six families of proteins within the GPCR superfamily.
Contact Information:Allen Lee
Vice President - Business Development
allensl@genetex.com
(949)553-1900
Original Source: GeneTex Augments Its GPCR Reagent Portfolio With a Highly Specific CXCR2 Recombinant Monoclonal Antibody
Editor Details
-
Company:
- Newswire
