
Global Viral Vector Development Market is predicted to achieve a US$ 4.1 Billion by 2033, mainly fueled by gene therapy solutions, according to FMI
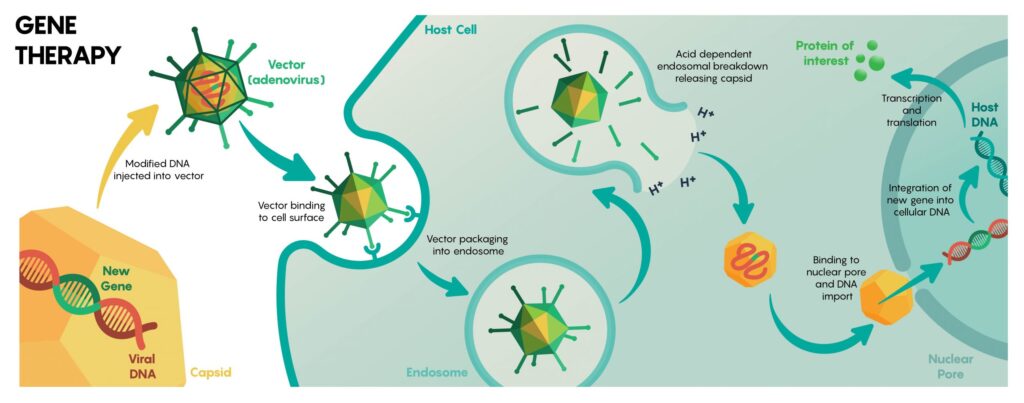 Viral Vector Development Market
Viral Vector Development Market
Over the evaluation period from 2023 to 2033, the global viral vector development market is predicted to grow at a remarkable CAGR of 18.7%. By the end of 2023, the global market is anticipated to be valued at US$ 730.7 million, rising to US$ 4.1 billion by 2033. The historical study shows that from 2017 to 2022, the global viral vector development market grew at a CAGR of 14.5%. Adeno-associated viral vectors (AAV), which are predicted to have a market share of roughly 37.0% worldwide in 2023, are currently leading the industry, according to recent studies by FMI. In 2022, the market for viral vector development was expected to be worth around 35.5% of the total US$30.86 million market for cancer gene therapy.
Since viral vectors are created in such a way that they utilize animal cell cultures. Furthermore, in some cases, insect cell cultures are essentially made from viruses that naturally infect human or other mammalian cells. Over the last ten years, a tremendous advancement has been witnessed in manufacturing techniques for the development of clinical-grade viral vectors. These techniques are useful for gene therapy methods in order to treat congenital or acquired disorders. AAV and lentiviral vectors are currently being used more frequently for in vivo and ex vivo gene delivery, respectively. Traditional medicine researchers and the medical world believe that hereditary disorders are incurable. Whereas, genetic therapy, on the other hand, heralded a new era in medicine by offering the chance to fix the damaged genes that cause certain inherited illnesses.
Get Recently Updated Report of Market as Sample Copy! https://www.futuremarketinsights.com/reports/sample/rep-gb-14415
Adeno-associated virus, often known as AAV, is frequently utilized as a carrier within the gene therapy procedure. The products made with viral vectors are offering suitable assistance for the market. Gene therapy comprises numerous potential applications in the medical sciences. Many biopharmaceutical companies are engaged in manufacturing and supply, which could be aimed at balancing the demand-supply ratio. Despite the unexpected disruption of the world economy for all products over the outbreak of flu season, it offered tremendous chances for the viral vector CDMO business to expand globally. The search for a therapeutic approach and a vaccine to prevent the causal pathogen’s rapid spread involved numerous research centers and private biopharmaceutical businesses.
Key Takeaways:
- Gene therapy is vital to repair suppressed and malfunctioning genes in human cells or tissues and helps in returning the abnormality to a normal level. Viral vector technology is commonly utilized to cultivate the gene. Furthermore, this business has grown for many private players. For the most part, two different types of techniques are used to use viral vectors in the gene therapy-induced healing process.
- When it comes to 2D planer technologies, scaling out of adherent cell systems is one of the common strategies that is applied until the point of market analysis. The production of suspension AAV by diverse segments will, therefore, rely increasingly on 3D suspension cell cultures or bioreactors in the upcoming period.
- The propelling synthesis of viral vectors utilizing cell culture technology has been modified successfully in order to satisfy the demands of both early and advanced clinical trial phases. Scale-up, however, may still be constrained depending on the vector type and the cell culture production platforms chosen.
- At present, visible progress is being made within the generation of cell lines that are able to create inducible or constitutively expressed lentiviral vectors that grow in suspension. Over the past few years, the first batch of lentiviral vectors generated by reliable producer cell lines was utilized in a clinical trial setting.
- With extensive safety and efficiency, the data gathered from numerous clinical trials show that gene therapy is making great progress. These factors are set to promote the expansion of the global market, over the forecast period.
Reach Out To Our Analyst And Get All Your Queries Answered! https://www.futuremarketinsights.com/ask-question/rep-gb-14415
Competitive Landscape:
Over recent years, the market for the production of plasmid DNA and viral vector CDMO has become extremely competitive. This has resulted in the introduction of numerous companies in these industries. The main tactic used by the leading firms to stay ahead of their rivals is the expansion of production facilities. A cost-effective strategy for the rapid expansion and capacity building for viral vector process development for the manufacturers is the acquisition of small regional companies.
Key Companies Covered:
- Thermo Fisher Scientific Inc
- Charles River (Cobra Biologics)
- NOVASEP
- uniQure N.V
- Waisman Biomanufacturing
- Creative Biogene
- GenScript Biotech Corporation
- Novartis AG
- Merck KGaA
- Takara Bio, Inc.
- FUJIFILM Diosynth Biotechnologies
- LONZA
- Danaher Corp. (Aldevron)
- Sirion Biotech GmbH
- AGC Biologics
More Insights into the Viral Vector Development Market:
The North American region is anticipated to dominate the global viral vector development market over the assessment period as it accounted for a total of 33.1% in 2022. The USA is expected to continue to grow its dominance in the global market within the next decade.
Personalized Experiences Redefined: Our Customization Report Holds the Answers! https://www.futuremarketinsights.com/customization-available/rep-gb-14415
Key Market Segments Covered in Viral Vector Development Industry Research:
Virus:
- Lentiviral Vectors
- Adenoviral Vectors
- Adeno-Associated Viral Vectors
- Retrovirus
Expression System:
- Transient
- Stable
Application:
- Gene Therapy
- Vaccines
- Cancer Therapy
- Others
End User:
- Biotechnology Companies
- Pharmaceutical Companies
- Contract Research Organization (CRO)
- Academic and Research Institutes
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 5000 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-845-579-5705
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Editor Details
-
Company:
- MARKITWIRED
- Website:
