
Rapid Growth at Attune Medical Spurs Strategic Hires and Expansion
Attune adds VP of Regulatory Affairs, Director of Clinical Research, and Marketing Manager to its ranks and expands commercial team
CHICAGO--(BUSINESS WIRE)--Attune Medical, a pioneer in utilizing the esophageal space to proactively manage patient temperature and to reduce the likelihood of esophageal injury during cardiac ablation procedures, announces significant growth with three key additions and expansion of commercial operations.



- Melinda Smith, MS, RAC, CBA, joins Attune Medical as VP of Regulatory Affairs leading our global regulatory strategy. Melinda brings over 20 years of medical device regulatory and quality experience along with a proven track record liaising with global regulatory bodies and expanding regulatory geographical footprints. She previously served as Director of Regulatory Affairs and Quality at Elekta, and Vice President, Quality and Regulatory Affairs at NeoMed, Inc.
- Tiffany Sharkoski, MPH, MBE, joins Attune Medical as Director of Clinical Research after serving for 20 years at the University of Pennsylvania, most recently as Associate Director of the Cardiovascular Clinical Research Unit. In that role she began working with Attune Medical in one of the first randomized controlled trials of the ensoETM, paving the way for numerous subsequent studies quantifying benefits in the safety, efficacy, and efficiency of esophageal cooling. In her role with Attune Medical, she will expand efforts in clinical research and study start-up while managing high-profile NIH funded clinical trial efforts.
- Luke Edmunds, BS, joins Attune Medical as Marketing Manager with extensive marketing and IT experience, heading the Digital Strategy & Marketing department at Danolyte Global and working in IT Recruitment at Allied One Source. Luke also worked in the Office of the President at Teva Pharmaceutical Industries. At Attune, Luke will be responsible for driving marketing strategy and leveraging the company’s new FDA marketing authorization to increase physician leads and awareness of the ensoETM.
In addition, the company is actively expanding its commercial and clinical teams to accommodate the rapid growth it is experiencing.
“I’m excited to welcome these talented individuals to our rapidly growing team,” commented Jay Istvan, CEO. “With the continued growth in ablative treatments for atrial fibrillation, we have seen a tremendous increase in demand for our technology. With these new additions to our team, Attune Medical will ensure that we can continue to effectively help patients and clinicians around the world.”
Following several recent growth milestones, including De Novo marketing authorization from the US FDA for use of its ensoETM to reduce the likelihood of esophageal injury during cardiac ablation procedures in September 2023, the company has shipped over 60,000 devices to over 200 hospitals and electrophysiology labs.
“Having used Attune Medical’s technology for over 4 years now, our group has seen not only the safety benefits, but also shorter procedure times, reduced fluoroscopy requirements, and greater hospital savings,” said Jason Zagrodzky, MD, FHRS of Texas Cardiac Arrhythmia in Austin, Texas. "I'm excited that this technology has become the standard of care for protecting patients against a potentially catastrophic complication.”
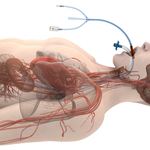
About ensoETM
Attune Medical’s ensoETM is a single use thermal regulating device that is placed in the esophagus (similar to a standard orogastric tube) and connected to an external heat exchange unit, creating a closed-loop system for proactive controlled temperature management.
About Attune Medical
Attune Medical’s novel technology platform pioneered the practice of using the esophageal space to proactively manage patient temperature and to reduce the likelihood of esophageal injury during cardiac ablation procedures.
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number R44HL158375. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Contacts
Lisa Owens, lisammowens@gmail.com, 210.601.6647
Editor Details
-
Company:
- Businesswire
