
In-Vitro Diagnostics Market Projected to Reach USD 116.5 Billion by 2032, Growing at 5.3% CAGR | Marketresearch.biz
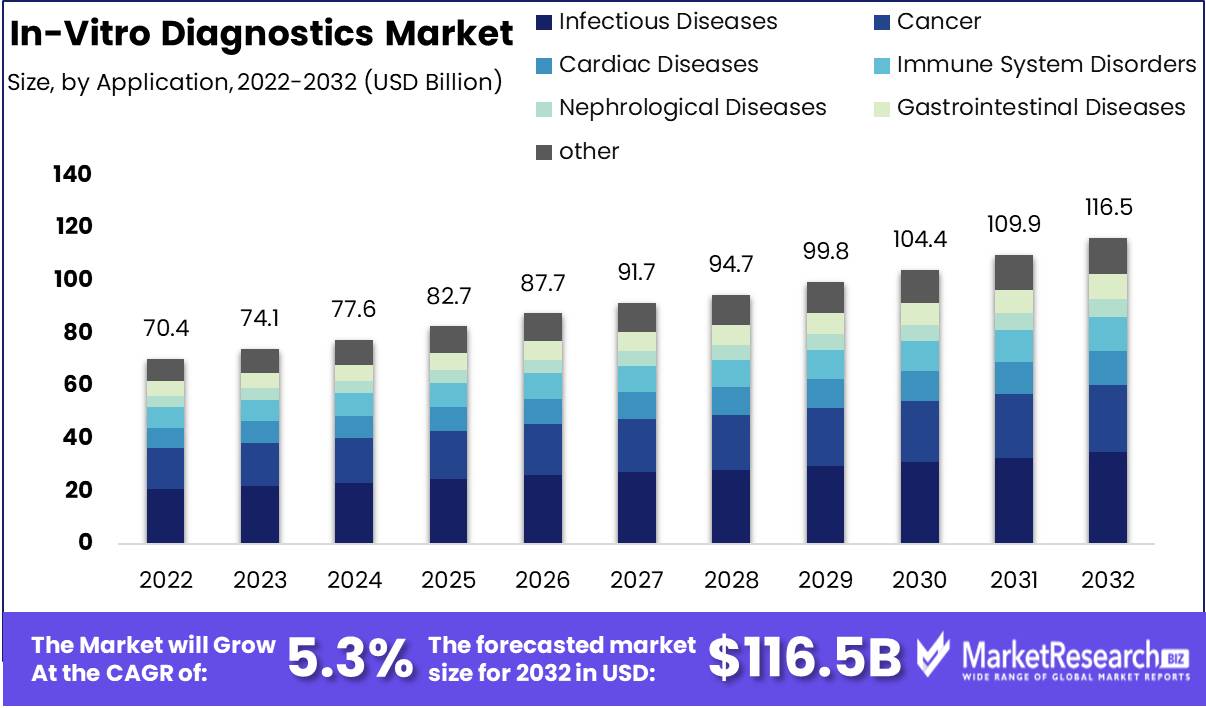
Marketresearch.biz reports that the In-Vitro Diagnostics Market size is expected to be worth around USD 116.5 Bn by 2032 from USD 70.4 Bn in 2022, growing at a CAGR of 5.3% during the forecast period from 2023 to 2032.
Overview of the In Vitro Diagnostics Market
In vitro diagnostics (IVD) play a crucial role in the detection and management of diseases by analyzing samples taken from the human body. This market encompasses a wide range of tests and technologies used for diagnosing conditions such as infections, cancers, diabetes, and cardiovascular diseases.
Get Full PDF Sample Copy of Report (Including Full TOC, List of Tables & Figures, Chart) Click Here to Download a Sample Report: https://marketresearch.biz/report/in-vitro-diagnostics-market/request-sample/
Driving Factors of the In Vitro Diagnostics Market
- Rising Disease Prevalence: The increasing incidence of chronic and infectious diseases worldwide drives the demand for accurate diagnostic tools.
- Technological Advancements: Continuous innovations in diagnostic technologies, including molecular diagnostics and point-of-care testing, enhance accuracy and efficiency.
- Growing Aging Population: With aging populations, there is a higher demand for diagnostic tests to monitor and manage age-related health conditions.
- Increasing Demand for Personalized Medicine: Tailoring treatments to individual patients’ genetic makeup requires precise diagnostic tools, driving market growth.
- Government Initiatives: Supportive government policies, funding for healthcare infrastructure, and initiatives promoting preventive healthcare fuel market expansion.
- Rapid Adoption of Point-of-Care Testing: The convenience and quick results offered by point-of-care testing drive its adoption, especially in resource-limited settings.
Restraining Factors of the In Vitro Diagnostics Market
- Regulatory Challenges: Stringent regulations for product approval and quality control processes can hinder market entry for new diagnostic technologies.
- Reimbursement Issues: Inadequate reimbursement policies for certain diagnostic tests may limit their adoption, particularly in developing regions.
- High Cost of Advanced Technologies: The high initial investment required for advanced diagnostic equipment and assays can pose financial barriers for healthcare providers and patients.
In conclusion, while the in vitro diagnostics market is driven by factors such as technological advancements and increasing disease prevalence, challenges related to regulation, reimbursement, and cost need to be addressed for sustainable growth and accessibility.
You can check In-Detail TOC from here: https://marketresearch.biz/report/in-vitro-diagnostics-market/
The In Vitro Diagnostics Market report provides a comprehensive exploration of the sector, categorizing the market by type, application, and geographic distribution. This analysis includes data on market size, market share, growth trends, the current competitive landscape, and the key factors influencing growth and challenges. The research also highlights prevalent industry trends, market fluctuations, and the overall competitive environment.
This document offers a comprehensive view of the Global In Vitro Diagnostics Market, equipping stakeholders with the necessary tools to identify areas for industry expansion. The report meticulously evaluates market segments, the competitive scenario, market breadth, growth patterns, and key drivers and constraints. It further segments the market by geographic distribution, shedding light on market leadership, growth trends, and industry shifts. Important market trends and transformations are also highlighted, providing a deeper understanding of the market’s complexities. This guide empowers stakeholders to leverage market opportunities and make informed decisions. Additionally, it provides clarity on the critical factors shaping the market’s trajectory and its competitive landscape.
Following Key Segments Are Covered in Our Report
By products and services
- Reagents & kits
- Instruments
- Services
By techniques
- Clinical chemistry
- Basic metabolic panel
- Electrolyte panel
- Liver panel
- Lipid profile
- Renal profile
- Thyroid function panel
- Specialty chemical tests
- Immunochemistry/Immunoassay
- Enzyme-linked immunosorbent assay (ELISA)
- Radioimmunoassays (RIA)
- Rapid tests
- Western blot
- ELISPOT
- Hematology
- Coagulation & hemostasis
- Clinical microbiology
- Molecular diagnostics (MDx)
- Polymerase chain reaction (PCR)
- Isothermal nucleic acid amplification technology (INAAT)
- Microarrays
- Hybridization
- DNA sequencing & next-generation sequencing
- Other MDX techniques
- Other techniques
By application
- Infectious Diseases
- Cancer
- Cardiac Diseases
- Immune System Disorders
- Nephrological Diseases
- Gastrointestinal Diseases
- Other applications
By end-user:
- Hospitals
- Laboratories
- Academic Institutes
- Other end users (Point-Of-Care testing and Patient self-testing)
Top Key Players in In Vitro Diagnostics Market
- Roche Diagnostics
- Siemens Healthineers
- Abbott Laboratories
- Danaher Corporation
- Thermo Fisher Scientific
- Becton, Dickinson and Company (BD)
- Bio-Rad Laboratories
- Ortho Clinical Diagnostics
- bioMérieux SA
- Quidel Corporation
- Sysmex Corporation
- PerkinElmer, Inc.
- Hologic, Inc.
- F. Hoffmann-La Roche Ltd
- Cepheid (a Danaher Company)
Get Full PDF Sample Copy of Report (Including Full TOC, List of Tables & Figures, Chart) Click Here to Download a Sample Report: https://marketresearch.biz/report/in-vitro-diagnostics-market/request-sample/
Regional Analysis of In Vitro Diagnostics Market
- North America: North America dominates the in vitro diagnostics market with a robust regulatory framework and high healthcare spending, particularly in the United States, driving demand for diagnostic reagents and instruments.
- Europe: Europe witnesses increasing adoption of in vitro diagnostics for disease management and personalized medicine, with significant consumption in countries like Germany, the UK, and France for clinical chemistry and immunoassay tests.
- Asia Pacific: Asia Pacific is a rapidly expanding market for in vitro diagnostics, fueled by growing healthcare infrastructure and rising prevalence of infectious diseases, especially in countries like China, India, and Australia.
- Middle East: The Middle East in vitro diagnostics market benefits from government initiatives for healthcare modernization and improving laboratory services, with demand driven by countries like Saudi Arabia and the UAE.
- Africa: Africa’s in vitro diagnostics market shows potential with increasing access to healthcare and efforts to combat infectious diseases, offering opportunities for market growth and adoption of point-of-care testing solutions.
For More Information or Qurey, Visit @ https://marketresearch.biz/report/in-vitro-diagnostics-market/
Growth Opportunities in In Vitro Diagnostics Market
- Expanding applications of in vitro diagnostics across various disease areas (e.g., infectious diseases, autoimmune disorders).
- Increasing adoption of point-of-care testing for rapid and efficient diagnosis.
- Rising geriatric population driving demand for diagnostic solutions.
- Technological advancements leading to the development of more sensitive and specific diagnostic assays.
- Growing demand for companion diagnostics in personalized medicine.
Trending Factors in In Vitro Diagnostics Market
- Shift towards digital pathology and remote diagnostics for improved accessibility and efficiency.
- Focus on automation and robotics to streamline laboratory workflows and reduce human error.
- Increasing utilization of biomarkers for early disease detection and monitoring.
- Integration of data analytics and cloud-based platforms for data management and analysis.
- Strategic partnerships and collaborations for product development and market expansion.
Our comprehensive Market research report endeavors to address a wide array of questions and concerns that stakeholders, investors, and industry participants might have. The following are the pivotal questions our report aims to answer:
Industry Overview:
- What are the prevailing global trends in the In Vitro Diagnostics Market?
- How is the In Vitro Diagnostics Market projected to evolve in the coming years? Will we see a surge or a decline in demand?
Product Analysis:
- What is the anticipated demand distribution across various product categories within In Vitro Diagnostics?
- Which emerging products or services are expected to gain traction in the near future?
Financial Metrics:
- What are the projections for the global In Vitro Diagnostics industry in terms of capacity, production, and production value?
- Can we anticipate the estimated costs, profits, Market share, supply and consumption dynamics?
- How do import and export figures factor into the larger In Vitro Diagnostics Market landscape?
Strategic Developments:
- What strategic initiatives and movements are predicted to shape the industry in the medium to long run?
Pricing and Manufacturing:
- Which factors majorly influence the end-price of In Vitro Diagnostics products or services?
- What are the primary raw materials and processes involved in manufacturing within the In Vitro Diagnostics sector?
Market Opportunities:
- What is the potential growth opportunity for the In Vitro Diagnostics Market in the forthcoming years?
- How might external factors, like the increasing use of In Vitro Diagnostics in specific sectors, impact the Market’s overall growth trajectory?
Historical Analysis:
What was the estimated value of the In Vitro Diagnostics Market in previous years, such as 2022?
Key Players Analysis:
- Who are the leading companies and innovators within the In Vitro Diagnostics Market?
- Which companies are positioned at the forefront and why?
Innovative Trends:
- Are there any fresh industry trends that businesses can leverage for additional revenue generation?
Market Entry and Strategy:
- What are the recommended Market entry strategies for new entrants?
- How should businesses navigate economic challenges and uncertainties in the In Vitro Diagnostics Market?
- What are the most effective Marketing channels to engage and penetrate the target audience?
Geographical Analysis:
- How are different regions performing in the In Vitro Diagnostics Market?
- Which regions hold the most potential for future growth and why?
Consumer Behavior:
- What are the current purchasing habits of consumers within the In Vitro Diagnostics Market?
- How might shifts in consumer behavior or preferences impact the industry?
Regulatory and Compliance Insights:
- What are the existing and upcoming regulatory challenges in the In Vitro Diagnostics industry?
- How can businesses ensure consistent compliance?
Risk Analysis:
- What potential risks and uncertainties should stakeholders be aware of in the In Vitro Diagnostics Market?
External Impact Analysis:
- How are external events, such as geopolitical tensions or global health crises (e.g., Russia-Ukraine War, COVID-19), influencing the In Vitro Diagnostics industry’s dynamics?
- This report is meticulously curated to provide a holistic understanding of the In Vitro Diagnostics Market, ensuring that readers are well-equipped to make informed decisions.
About Company
MarketResearch .Biz, a division of Prudour Pvt Ltd, excels in providing thorough Market research and analytical services. With a strong history of reliability, our company has established itself as a trusted consulting agency and a source for custom Market research insights. At MarketResearch .Biz, we recognize the diverse needs of our clients and are equipped to offer reports tailored to their specific requirements. Our dedication extends beyond standard practices, ensuring that we consistently deliver top-notch insights and a comprehensive view of the Market landscape to our clients.
Mr. Lawrence John
Marketresearch.Biz (Powered By Prudour Pvt. Ltd.)
420 Lexington Avenue, Suite 300
New York City, NY 10170,
United States
Tel: +1 (347) 796-4335
lawrence@marketresearch.biz
inquiry@marketresearch.biz
Editor Details
-
Company:
- Wired Release
- Website:
