
Deep Brain Stimulation Devices Market Expands, Aiming for USD 3.5 Billion Valuation by 2033
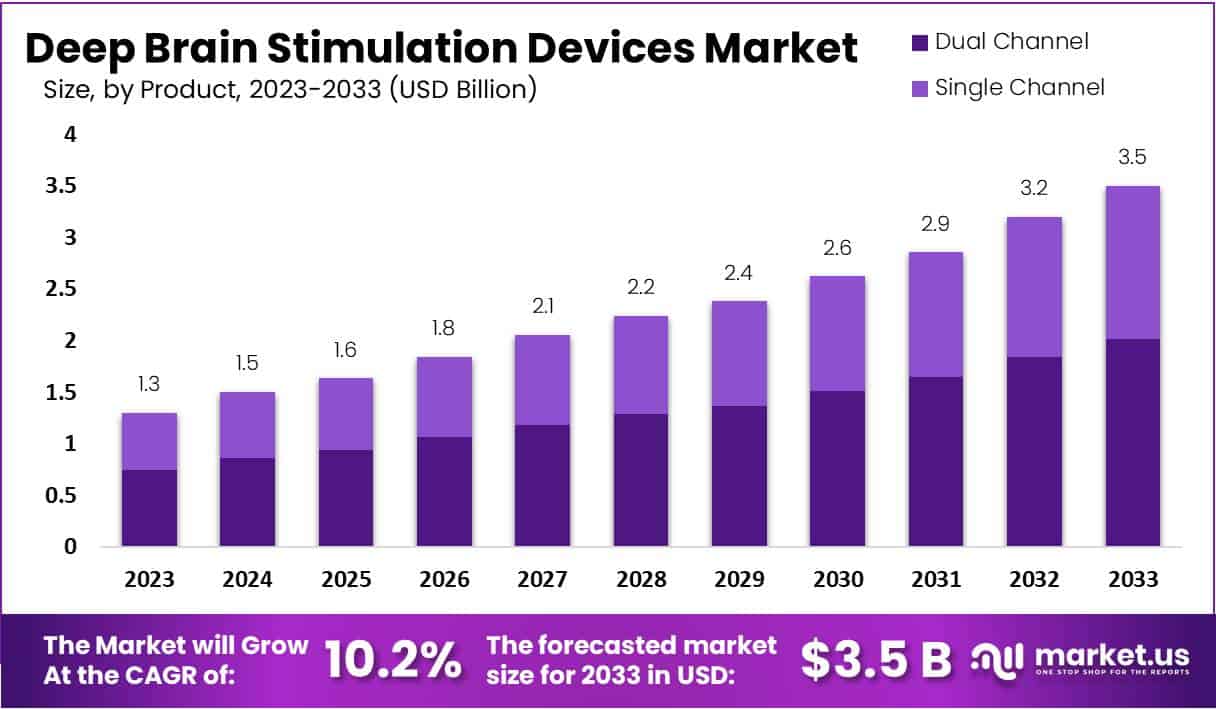
The Deep Brain Stimulation Devices Market is poised for considerable growth, projected to expand from USD 1.3 billion in 2023 to USD 3.5 billion by 2033, achieving a compound annual growth rate (CAGR) of 10.2% over this period. This growth is largely driven by the increasing incidence of neurological disorders such as Parkinson’s disease, which dominates the application segment due to its prevalence and the effectiveness of deep brain stimulation in managing its symptoms. The market is further bolstered by technological advancements in the devices themselves, which enhance their effectiveness and ease of use.
Challenges in this market include the high costs associated with these devices and the specialized skills required to operate them. However, the rising awareness and the aging global population are factors that contribute positively to the market dynamics. North America currently leads the market, supported by robust healthcare infrastructure and substantial investments in research and development. The region’s market share is followed by Europe, which is also experiencing significant growth due to a high incidence of chronic neurological disorders.
Recent developments include innovations such as ‘Smart’ Deep Brain Stimulation systems, which adjust stimulation based on patient symptoms, and new product approvals which are expanding the applications of these devices beyond traditional uses like Parkinson’s and epilepsy to include conditions such as obsessive-compulsive disorder. These advancements are expected to further drive the adoption and refinement of deep brain stimulation therapies, enhancing their appeal to both patients and healthcare providers across the globe.
Key Takeaways
- Projected market size for 2033 is estimated at USD 3.5 billion, up from USD 1.3 billion in 2023, indicating significant growth.
- Dual Channel devices currently dominate, holding a 57.5% market share, and are preferred for their advanced therapeutic benefits in treating neurological conditions.
- The Parkinson’s Disease application segment leads with a 65.8% market share, highlighting its significant impact within the sector.
- Hospitals & Clinics are the primary end-users, dominating the market with a 52.2% share, reflecting their pivotal role in deployment.
- Opportunities for market expansion are notable in emerging markets, propelled by increasing neurological disorders and enhanced healthcare infrastructures.
- In regional terms, North America is at the forefront with a 51.4% share, valued at USD 0.668 billion, due to ongoing innovations and superior healthcare facilities.
Get Sample PDF Report: https://market.us/report/deep-brain-stimulation-devices-market/request-sample/
Deep Brain Stimulation Devices Market Key Segments
Product
- Single Channel
- Dual Channel
Application
- Pain Management
- Epilepsy
- Essential Tremor
- Obsessive Compulsive Disorder (OCD)
- Parkinson’s Disease
- Depression
- Dystonia
- Others
End-User
- Hospitals & Clinics
- Homecare
- Others
Buy Directly: https://market.us/purchase-report/?report_id=17297
Key Players Analysis
- Abbott, through its acquisition of St. Jude Medical, has made significant advances in the Deep Brain Stimulation (DBS) sector, particularly with the Infinity DBS system. This system, which gained FDA approval and had its first implant in the U.S. in 2016, is notable for its directional lead technology that allows for precise symptom control in patients with Parkinson’s disease or essential tremor. The system is engineered to minimize side effects by directing stimulation away from sensitive brain areas. Moreover, it incorporates a first-of-its-kind wireless software platform operating on iOS, enhancing both the patient and physician experience through easy and discreet management of the therapy settings. The Infinity DBS system underscores Abbott’s commitment to improving patient outcomes in the treatment of movement disorders through innovative technological enhancements.
- Medtronic, a leading global healthcare technology company, has made significant advancements in the deep brain stimulation (DBS) devices sector. Their latest innovation, the Percept
 RC neurostimulator, has recently received FDA approval and CE Mark for use in Europe. This device stands out for its BrainSense
RC neurostimulator, has recently received FDA approval and CE Mark for use in Europe. This device stands out for its BrainSense technology, which allows for the monitoring and recording of brain activity, enabling personalized treatment for patients with neurological disorders such as Parkinson’s disease and epilepsy. The Percept
technology, which allows for the monitoring and recording of brain activity, enabling personalized treatment for patients with neurological disorders such as Parkinson’s disease and epilepsy. The Percept RC is noted for its compact design, long battery life, and rapid recharging capabilities, enhancing patient comfort and convenience. Medtronic’s commitment to technological advancement in DBS is supported by their extensive research and development, aiming to improve patient outcomes and therapy personalization.
RC is noted for its compact design, long battery life, and rapid recharging capabilities, enhancing patient comfort and convenience. Medtronic’s commitment to technological advancement in DBS is supported by their extensive research and development, aiming to improve patient outcomes and therapy personalization. - Boston Scientific Corporation is making significant strides in the Deep Brain Stimulation (DBS) devices sector, particularly with their Vercise Genus DBS Systems. These systems are designed to treat neurological conditions like Parkinson’s disease and essential tremor by delivering targeted electrical stimulation. The Vercise Genus DBS System, known for its advanced technology, allows clinicians to personalize therapy based on individual patient needs, utilizing its multiple independent current control and unique directional capabilities. Additionally, the Vercise Neural Navigator 5 Software enhances these systems by providing intuitive therapy management, which simplifies the programming process and improves patient outcomes. Boston Scientific’s continued innovation in this field highlights their commitment to developing technologies that improve the quality of life for patients with neurological disorders.
- Aleva Neurotherapeutics S.A., a Swiss company that emerged from the Swiss Federal Institute of Technology in Lausanne, specializes in innovative Deep Brain Stimulation (DBS) systems for neurological conditions like Parkinson’s disease. Their flagship product, directSTIM
 , integrates directional electrode technology that enhances the precision of stimulation, potentially reducing side effects and improving patient outcomes. This technology recently received CE marking, which supports its use in the European market, and has also been recognized with an MRI approval, allowing patients to safely undergo full-body scans post-implantation. Aleva’s strategic focus now includes expanding into U.S. markets, following successful pilot studies and sustained investment in their advanced DBS solutions.
, integrates directional electrode technology that enhances the precision of stimulation, potentially reducing side effects and improving patient outcomes. This technology recently received CE marking, which supports its use in the European market, and has also been recognized with an MRI approval, allowing patients to safely undergo full-body scans post-implantation. Aleva’s strategic focus now includes expanding into U.S. markets, following successful pilot studies and sustained investment in their advanced DBS solutions. - Nexstim is a pioneering company in the deep brain stimulation sector, primarily focusing on the development and application of navigated Transcranial Magnetic Stimulation (nTMS) systems. Their technology integrates 3D imaging to target the E-field precisely and monitor the stimulation effects in real-time, leveraging the individual’s MRI head scans for personalized treatment planning. Nexstim’s devices, notably the NBS System 5 and NexSpeech module, are approved by the FDA for motor and speech cortical mapping, crucial for pre-surgical planning in neurological procedures. This technology supports non-invasive mapping of brain areas, significantly enhancing the safety and efficacy of neurosurgical interventions. The integration of TMS with neuronavigation offers a significant advantage, providing accurate targeting that supports better clinical outcomes.
Deep Brain Stimulation Devices Market Key Players:
- Abbott (St. Jude Medical)
- Medtronic
- Boston Scientific Corporation
- Aleva Neurotherapeutics S.A.
- Nexstim
- LivaNova PLC
- Neuropace Inc.
Inquire More about report: https://market.us/report/deep-brain-stimulation-devices-market/#inquiry
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: inquiry@market.us
Blog: https://medicalmarketreport.com/
View More Trending Reports
Gene Therapy Market Valuation Expected To Hit USD 49.3 Billion By 2032, Demonstrating A 25% CAGR
Medical Tubing Market Will Reach USD 20.4 Billion By 2032 And Hit Around 7.8% CAGR
Enteral Feeding Devices Market Outlook: Expected To Expand To USD 6.7 Billion Upholding A 6.5% CAGR
Pharmacovigilance Market Projected To Reach USD 19 Billion By 2032, With CAGR Of 9.3%
Editor Details
-
Company:
- Wired Release
- Website:
