
Expanding Adalimumab Biosimilar Market to Achieve 23.4% CAGR Over the Next Decade
The Adalimumab Biosimilar Market is experiencing substantial growth, with projections indicating it will reach approximately USD 6044.9 million by 2033, up from USD 738.3 million in 2023. This increase represents a compound annual growth rate (CAGR) of 23.4% from 2024 to 2033. Key growth drivers include the rising prevalence of chronic diseases like rheumatoid arthritis and psoriasis, which are increasing the demand for cost-effective treatment options such as adalimumab biosimilars. Additionally, the expiration of patents for original adalimumab products has opened the market to biosimilar manufacturers, fostering competition and making treatments more affordable. Supportive regulatory environments also play a crucial role by streamlining the approval processes for these biosimilars.
However, the market faces challenges, including the complex manufacturing processes required for biosimilars, which can result in higher production costs and difficulties in maintaining consistent quality. Stringent regulatory requirements also pose barriers to market entry.
Recent developments have seen significant activities from major players. For instance, in October 2023, Pfizer’s adalimumab biosimilar, ABRILADA, received an interchangeable designation from the FDA, expected to launch at a reduced price point. Similarly, Boehringer Ingelheim’s CYLTEZO has been launched at an 81% discount to HUMIRA, highlighting the competitive pricing strategies being adopted in the market. Celltrion’s YUFLYMA also received FDA approval for new dosage forms, expanding its market presence.
These factors collectively indicate a dynamic and rapidly evolving market landscape for adalimumab biosimilars, driven by a mix of favorable regulatory policies, competitive pricing, and ongoing product innovations.
Key Takeaways
- Impressive Market Growth: The Adalimumab Biosimilar Market is projected to reach USD 6,044.9 million by 2033, with a CAGR of 23.4% from 2024 to 2033.
- Product Dominance: Exemptia commanded a 32.1% market share in 2023, attributed to its advanced formulation, efficacy, and competitive pricing.
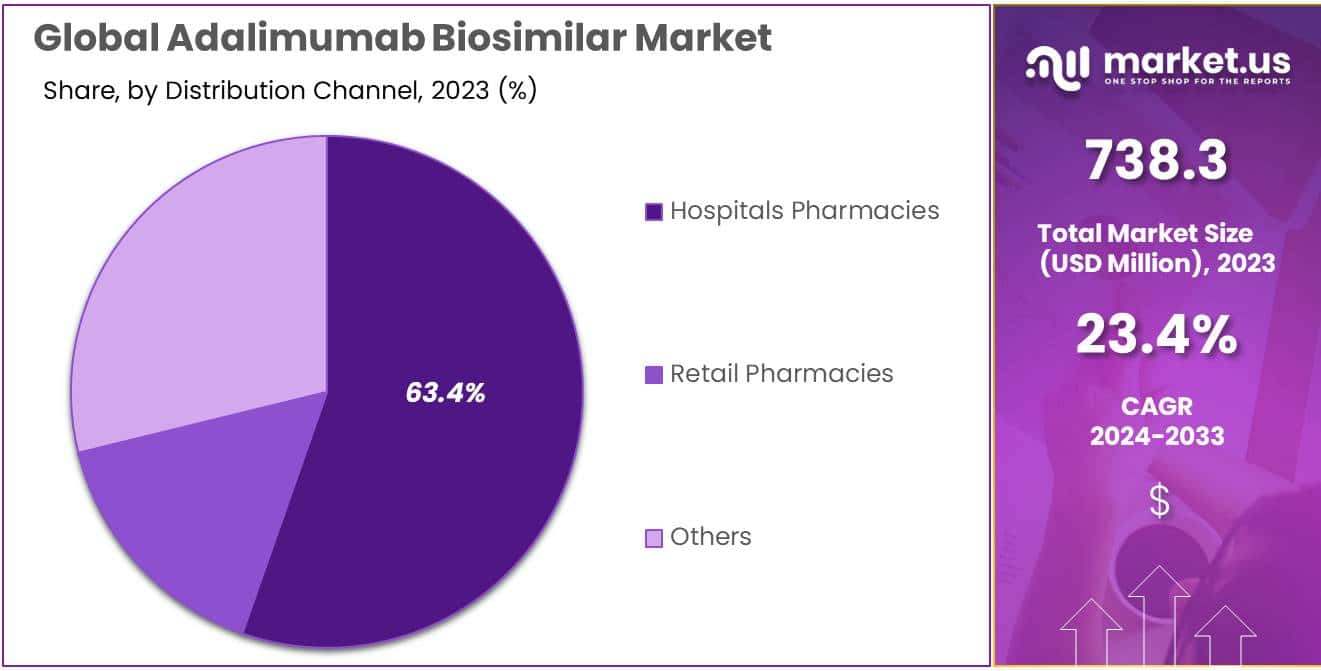
- Distribution Leadership: Hospital pharmacies led the market with over 32.1% share in 2023, due to convenient access within healthcare ecosystems.
- Retail Pharmacy Growth: Retail pharmacies showed steady growth, offering accessible sources for Adalimumab Biosimilar medications to patients.
- Driving Factors: Rising chronic diseases, patent expirations, cost-efficient healthcare, and supportive regulatory environments are key market drivers.
- Market Challenges: Complex manufacturing processes, stringent regulatory requirements, and limited patient understanding pose significant market expansion challenges.
- Promising Opportunities: Emerging market expansion, strategic collaborations, technological advancements, and diversified therapeutic indications offer substantial growth opportunities.
- Current Trends: Notable trends include biosimilar lifecycle management, patient assistance programs, unique naming strategies, and a shift towards self-administered biosimilars.
- Regional Insights: North America held a dominant 43.5% market share in 2023 (USD 172.7 million), with Asia Pacific showing the highest growth potential.
Get Sample PDF Report: https://market.us/report/adalimumab-biosimilar-market/request-sample/
Adalimumab Biosimilar Market Key Segments
Product
- Exemptia
- Adalirel
- Cipleumab
- Others
Distribution Channel
- Hospitals Pharmacies
- Retail Pharmacies
- Others
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=36720
Key Players Analysis
Alfred E. Tiefenbacher (AET) has a dedicated biosimilars business, AET BioTech, which is actively involved in the development of a biosimilar for Adalimumab, a TNF inhibitor originally marketed by Abbott. In collaboration with BioXpress Therapeutics, AET BioTech is focusing on the joint development, registration, and manufacture of this biosimilar. The partnership leverages BioXpress’s advanced monoclonal antibody (MAb) technology and AET BioTech’s expertise in drug development and commercialization, aiming to provide high-quality, cost-effective biosimilars globally.
Amgen Inc. has made significant strides in the adalimumab biosimilar sector with its product AMJEVITA . Approved by the FDA, AMJEVITA is the first biosimilar to AbbVie’s Humira, offering a cost-effective alternative for treating various inflammatory diseases. Launched in the U.S. in early 2023, AMJEVITA features a unique tiered pricing strategy, with discounts up to 55% off Humira’s list price, aimed at enhancing patient access and reducing healthcare costs. This approach has positioned Amgen as a key player in the competitive biosimilars market, providing broad access through innovative pricing and extensive patient support programs.
. Approved by the FDA, AMJEVITA is the first biosimilar to AbbVie’s Humira, offering a cost-effective alternative for treating various inflammatory diseases. Launched in the U.S. in early 2023, AMJEVITA features a unique tiered pricing strategy, with discounts up to 55% off Humira’s list price, aimed at enhancing patient access and reducing healthcare costs. This approach has positioned Amgen as a key player in the competitive biosimilars market, providing broad access through innovative pricing and extensive patient support programs.
Boehringer Ingelheim has made significant strides in the adalimumab biosimilar sector with its product, Cyltezo (adalimumab-adbm). As the first FDA-approved interchangeable biosimilar to Humira, Cyltezo is designed to treat multiple chronic inflammatory diseases, including rheumatoid arthritis, psoriasis, and Crohn’s disease. The approval was backed by robust clinical data, including the VOLTAIRE-X trial, which confirmed its safety and efficacy when switching between Humira and Cyltezo. This designation allows pharmacists to substitute it for Humira without needing to inform the prescribing physician, improving access and potentially lowering costs for patients.
Glenmark Pharmaceuticals has launched the biosimilar of Adalimumab, branded as ADALY, in India. This biosimilar, developed under a licensing agreement with Cadila Healthcare, is intended for the treatment of plaque psoriasis and rheumatoid arthritis. By introducing ADALY at a fraction of the global cost, Glenmark aims to enhance its presence in the dermatology segment and address unmet patient needs. This launch reflects Glenmark’s commitment to expanding its portfolio of biologics and reinforces its position as a leader in dermatology in India.
Zydus Group has developed Exemptia, the world’s first biosimilar of adalimumab, which is used to treat autoimmune diseases like rheumatoid arthritis and psoriatic arthritis. This biosimilar, launched in 2014, offers a cost-effective alternative to the original drug, reducing treatment costs significantly. Exemptia is part of Zydus’ extensive biologics program, which includes 24 biologics in its R&D pipeline. The drug’s affordability and effectiveness aim to make advanced biologic treatments accessible to a broader patient population.
Adalimumab Biosimilar Market Key Players:
- Alfred E. Tiefenbacher
- Amgen Inc.
- Boehringer Ingelheim International GmbH
- Glenmark
- Zydus Group
- Torrent Pharmaceuticals Ltd.
- Emcure Pharmaceuticals Ltd
- AET BioTech
- Coherus Biosciences
- Fujifilm Kyowa Kirin Biologics Co. Ltd.
Adalimumab Biosimilar Market Report Scope >> Market Value (2023): USD 738.3 Million || Forecast Revenue (2033): USD 6044.9 Million || CAGR (2024-2033): 23.4% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/adalimumab-biosimilar-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: inquiry@market.us
Blog: https://medicalmarketreport.com/
View More Trending Reports
Healthcare BPO Market Estimated To Reach USD 908 Billion By 2032, With Round About 9.9% CAGR
Dental 3D Printing Market Economic Projections Soar To USD 19.6 Billion By 2032, With 23.03% CAGR
Peptide Therapeutics Market Predicted USD 90 Billion By 2033, An Approximate 8.9% CAGR Growth
Editor Details
-
Company:
- Wired Release
- Website:
