
Clinical Trials Market Growth Accelerates: Expected CAGR of 7.2% from 2023 to 2032
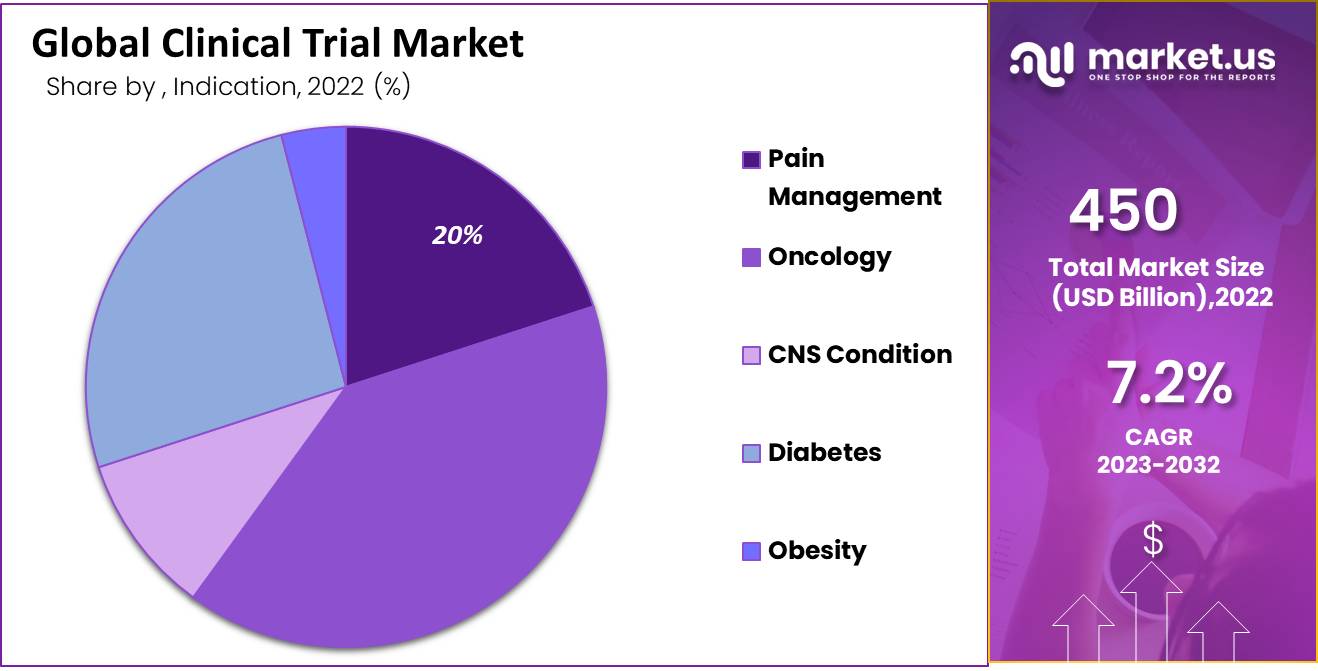
The global clinical trials market is projected to grow significantly, with an expected value of USD 886.5 billion by 2032, up from USD 450.1 billion in 2022, reflecting a compound annual growth rate (CAGR) of 7.2% from 2023 to 2032. This growth can be attributed to several factors, including the rising prevalence of chronic and rare diseases, increased investments in pharmaceutical research and development, and advancements in digital solutions and personalized medicine.
Growth in the clinical trials market is further driven by the increasing number of industry-sponsored trials, particularly in oncology and cardiovascular conditions, where substantial investments are being made. For instance, the pharmaceutical industry spends over USD 38 billion annually on the development of oncology therapies (Fortune Business Insights). Additionally, the adoption of decentralized clinical trials and advanced technologies such as artificial intelligence and machine learning has streamlined data management and patient recruitment processes.
However, the market faces several challenges, including high costs and prolonged approval periods for clinical trials, regulatory complexities, and difficulties in patient recruitment and retention. These challenges are particularly pronounced in the later phases of clinical trials, where extensive subject involvement and higher costs are common.
Recent developments in the clinical trials market include strategic alliances and acquisitions aimed at accelerating drug development and enhancing trial efficiencies. For example, in 2023, Eli Lilly partnered with Precision BioSciences to advance CAR-T cell therapies for cancer, and Syneos Health launched a new patient recruitment platform leveraging social media. Overall, the clinical trials market is poised for robust growth, supported by technological advancements, increasing disease prevalence, and ongoing innovations in trial methodologies.
Key Takeaways
- The Clinical Trials Market is projected to reach USD 886.5 billion by 2032, growing at a CAGR of 7.2% from 2022 to 2032.
- The market was valued at USD 450.1 billion in 2022, driven by increased demand in developing countries and technological advancements.
- An aging population and globalization of clinical trials contribute to market growth.
- Online resources are enhancing patient recruitment rates for clinical trials.
- The globalization of drug development and rising chronic disease cases boost market expansion.
- High costs of clinical trials and extended approval periods hinder market growth.
- Clinical research organizations (CROs) are in demand, providing significant growth opportunities.
- Lack of skilled personnel poses a challenge to market growth.
- Phase III trials generate the highest revenue, followed by Phase II trials.
- Phase II trials are crucial for oncology studies and COVID-19 treatment development.
- Companies are collaborating on therapeutics and vaccines for COVID-19.
- The oncology segment leads in revenue, with cardiovascular conditions expected to grow.
- The pandemic disrupted clinical trials, reducing patient enrollment.
- Pharmaceutical and biopharmaceutical companies dominate the market, with growing demand for CROs.
- Outsourcing clinical trials is a rising trend, benefiting the market.
- Budget constraints and lack of foreign direct investment limit market growth.
- Government initiatives and advanced technology drive market expansion.
- North America leads the market due to new technologies and R&D investments.
- Asia-Pacific is growing rapidly due to the pandemic and a large patient pool.
- Key players include Eli Lilly, Parexel, Pfizer, and IQVIA.
Get Sample PDF Report: https://market.us/report/clinical-trials-market/request-sample/
Clinical Trials Market Key Segments
Based on Phase
- Phase I
- Phase II
- Phase III
- Phase IV
Based on Indication
- Pain Management
- Oncology
- CNS Condition
- Diabetes
- Obesity
Based on End-User
- Pharmaceutical and Biopharmaceutical Companies
- Clinical Research Organizations
- Healthcare Providers
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=66238
Key Players Analysis
Eli Lilly is a leading player in the clinical trials sector, focusing on innovative therapies for conditions such as diabetes, cancer, and neurodegenerative diseases. Their recent advancements include the AK-OTOF-101 trial, a gene therapy for restoring hearing in patients with genetic hearing loss. This trial has shown promising results, highlighting the potential of genetic medicines. Eli Lilly is also known for its diverse pipeline, with significant investments in biopharmaceuticals and personalized medicine, aiming to improve patient outcomes globally.
Parexel International Corporation is a prominent contract research organization (CRO) that provides comprehensive drug development and regulatory consulting services. Parexel is known for its expertise in conducting Phase I-IV clinical trials across various therapeutic areas, including oncology, neurology, and rare diseases. Their commitment to patient-centric approaches and innovative technologies, such as decentralized clinical trials, has positioned them as a key player in accelerating drug development and ensuring regulatory compliance.
Pfizer is a global leader in the pharmaceutical industry, renowned for its extensive clinical trial network. The company has been pivotal in developing and testing vaccines, most notably the COVID-19 vaccine. Pfizer’s clinical trials span multiple therapeutic areas, including oncology, immunology, and cardiovascular diseases. Their approach integrates cutting-edge technologies and data analytics to streamline trial processes, enhance patient recruitment, and ensure robust data collection, contributing significantly to medical advancements.
Charles River Laboratories specializes in providing essential preclinical and clinical laboratory services to support the pharmaceutical and biotechnology industries. They offer comprehensive services, including safety assessment, laboratory animal models, and regulatory support. Charles River’s expertise in early-stage drug development and non-clinical testing helps accelerate the translation of novel therapies from the laboratory to clinical trials, ensuring safety and efficacy before human testing.
Syneos Health is a leading biopharmaceutical solutions organization that integrates clinical development and commercial services. They are known for their full-service capabilities, managing clinical trials from Phase I through IV and providing commercialization strategies. Syneos Health’s approach combines therapeutic expertise with innovative technologies to enhance trial efficiency and patient engagement. Their global reach and collaborative model support the development and delivery of new therapies to market.
Clinical Trials Market Key Players:
- Eli Lilly and Company
- Parexel International Corporation
- Pfizer
- Charles River Laboratory
- Syneous Health
- Novo Nordisk A/S
- IQVIA
- ICON Plc.
- Other Key Players.
Clinical Trials Market Report Scope >> Market Value (2022): USD 450.1 Billion || Forecast Revenue (2032): USD 886.5 Billion || CAGR (2023-2032): 7.2% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/clinical-trials-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: inquiry@market.us
Blog: https://medicalmarketreport.com/
View More Trending Reports
Gene Therapy Market Valuation Expected To Hit USD 49.3 Billion By 2032, Demonstrating A 25% CAGR
Legal Marijuana Market Will Increase USD 86.3 Billion By 2033 And Has Guessed Around 16.1% CAGR
Influenza Vaccine Market Will Increase USD 13.9 Billion By 2033 With Almost 7.58% CAGR
Biobanking Market Forecasted To Attain USD 88.7 Billion By 2032, Showcasing A 6.3% CAGR
Editor Details
-
Company:
- Wired Release
- Website:
