
North America Leads $12.2+ Billion Medical Device Regulatory Affairs Market

In 2021, the global medical device regulatory affairs market demonstrated its robustness with a valuation of $7.0 billion. Looking ahead, projections indicate a significant surge, with estimations pointing towards a staggering $12.2 billion by 2031. This growth trajectory illustrates a steady compound annual growth rate (CAGR) of 5.8% from 2022 to 2031. Such expansion underscores the increasing importance and complexity of regulatory affairs in the realm of medical devices, reflecting the evolving landscape of healthcare regulations and the demand for compliance and quality assurance.
Get a Sample Copy of this Report: https://www.alliedmarketresearch.com/request-sample/A16307
- Importance of Regulatory Function in Healthcare Industry:
- Critical for ensuring safety and efficacy of healthcare products globally.
- Involves regulatory compliance, submissions, clinical affairs, and quality assurance.
- Medical device regulatory affairs experts facilitate communication between industry and regulatory bodies worldwide.
- Significance of Regulatory Compliance for Medical Devices:
- Development of medical devices entails significant costs; errors can tarnish company reputation.
- Vital for diagnosis, prevention, and treatment of diseases.
- Regulatory affairs professionals ensure conformity and accurate communication of device information to patients.
- Market Growth Drivers and Opportunities:
- Moderate growth expected due to advanced medical device adoption and technological advancements.
- Opportunities arise from aging population and technological innovations meeting unmet patient needs.
- Challenges in Market Growth:
- High costs of regulatory services and cybersecurity threats for software-based medical devices pose challenges.
- Segmentation of Medical Device Regulatory Affairs Market:
- Services: Regulatory consulting, writing & publishing, legal representation, product registration & clinical trials.
- Service Providers: In-house and outsourcing.
- Types: Diagnostics and therapeutics.
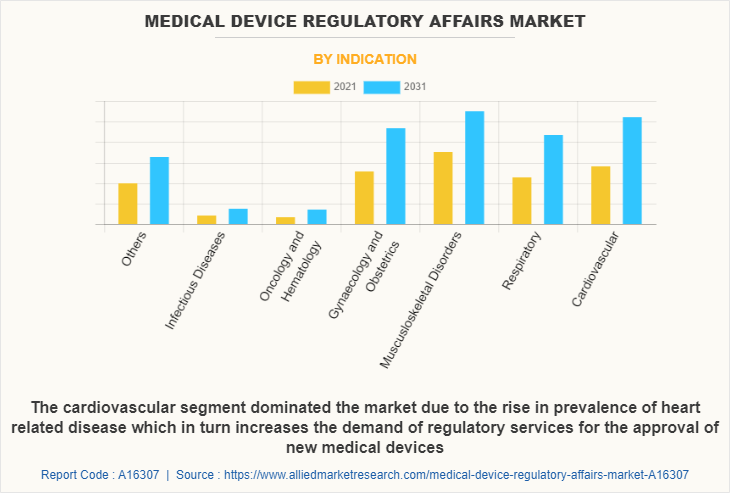
- Indications: Infectious diseases, oncology & hematology, musculoskeletal disorders, cardiovascular, etc.
- Regions: North America, Europe, Asia-Pacific, and LAMEA.
- Revenue Contributions and Growth Projections:
- Legal representation and product registration & clinical trials segments show significant revenue and growth.
- Outsourcing and in-house service providers both exhibit growth, catering to different industry needs.
- Therapeutics segment dominates revenue due to demand for advanced therapeutic products.
- Diagnostic segment poised for growth due to regulatory allowances for certain equipment.
- Musculoskeletal disorders and cardiovascular segments lead in revenue contribution and growth.
- Regional Analysis:
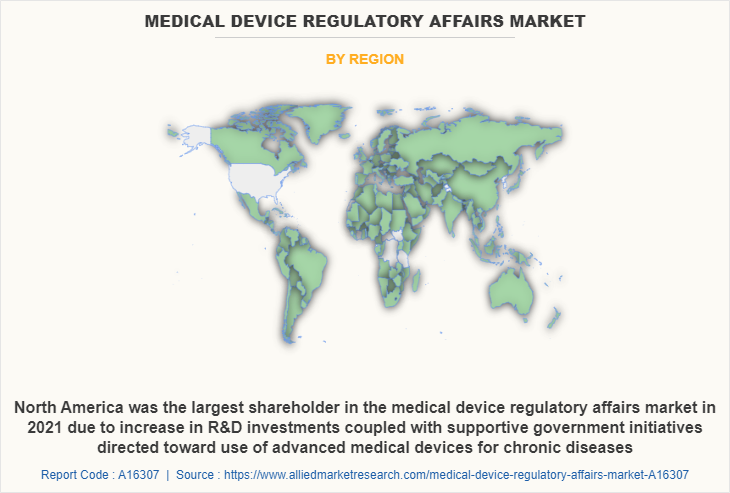
- North America leads market share due to aging population and government support.
- Asia-Pacific market expected to grow, driven by product approvals, clinical trials, and demand for advanced devices.
 Enquire Before Buying: https://www.alliedmarketresearch.com/purchase-enquiry/A16307
Enquire Before Buying: https://www.alliedmarketresearch.com/purchase-enquiry/A16307
Editor Details
-
Company:
- The Wire Times
