
Biological Safety Testing Market Growth with a CAGR of 10.9% Through 2033
The Biological Safety Testing Market is set to experience significant growth, with its market size projected to reach approximately USD 13.4 billion by 2033, up from USD 4.8 billion in 2023. This expansion, driven by a Compound Annual Growth Rate (CAGR) of 10.9% from 2024 to 2033, reflects the increasing demand for rigorous safety assessments in the pharmaceutical and biotechnology sectors.
Several factors contribute to this robust growth. The rising prevalence of chronic diseases and the subsequent surge in biopharmaceutical production have heightened the need for comprehensive safety testing. The development and widespread adoption of novel biologics, such as gene and cell therapies, require stringent testing to ensure product safety and efficacy. Additionally, the global focus on vaccine development, particularly in response to pandemics like COVID-19, has further propelled market demand. Regulatory guidelines and the need for compliance with safety standards also play a crucial role in driving market growth.
However, the market faces several challenges. High costs associated with advanced testing technologies and stringent regulatory requirements can impede market expansion. Moreover, the complexity of biological products necessitates continuous advancements in testing methodologies, which can be resource-intensive for companies.
Recent developments in the market include significant technological advancements and strategic collaborations. For instance, Charles River Laboratories launched the Endosafe Trillium rCR cartridge, an animal-free testing solution for endotoxin testing, and Merck KGaA expanded its biosafety testing laboratories in Shanghai to support local biopharmaceutical clients. These innovations and partnerships are expected to enhance testing capabilities and support the growing market demand for biological safety testing services.
The Biological Safety Testing Market is poised for substantial growth, driven by increased biopharmaceutical production, advancements in biologics, and a global emphasis on vaccine safety, despite facing challenges related to costs and regulatory compliance.
Key Takeaways
- Market Growth: The Biological Safety Testing Market is projected to reach USD 13.4 billion by 2033, with a 10.9% CAGR from 2024 to 2033.
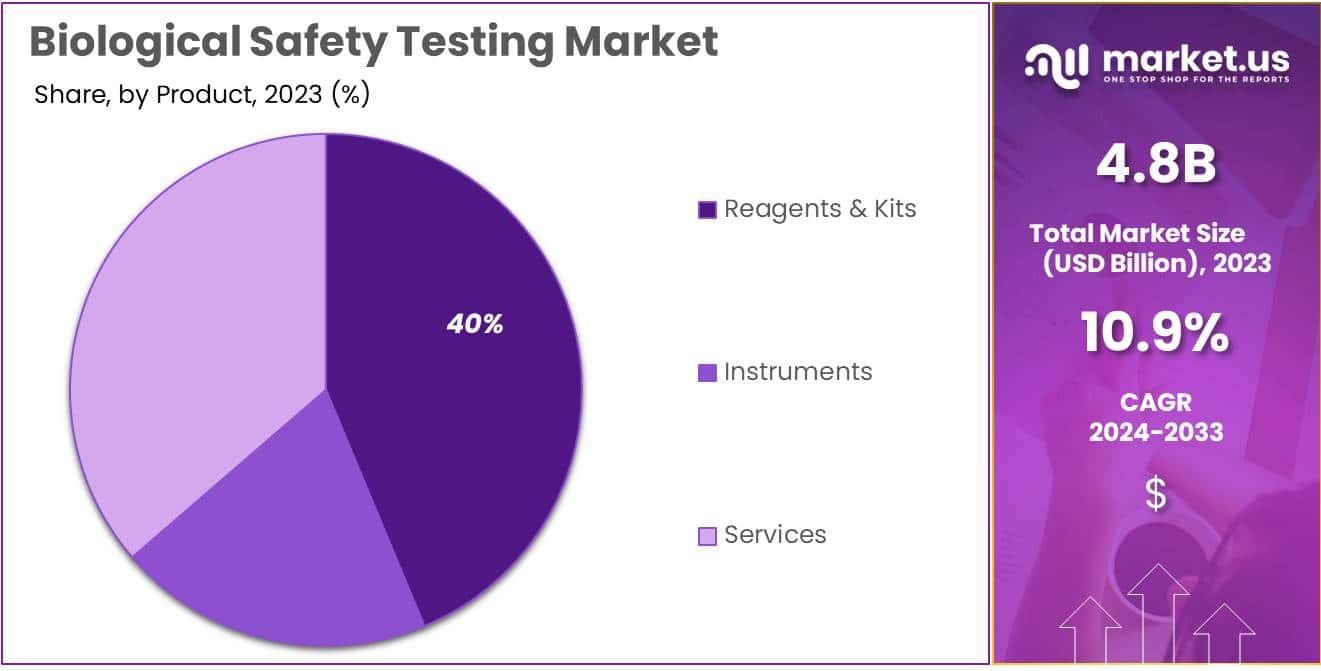
- Product Dominance: Reagents & Kits dominated in 2023 with over 39.7% market share, essential for accurate biological safety testing.
- Application Dominance: Vaccines & Therapeutics held a 24.1% share in 2023, highlighting the importance of safety testing in medical interventions.
- Test Type Dominance: Endotoxin Tests led in 2023 with over 22.6% share, ensuring safety by detecting harmful endotoxins.
- Key Driver – R&D Focus: Increased financial support for innovative biologics and biosimilars is driving market growth.
- Industry Expansion: Global growth in the pharmaceutical and biotechnology industries is boosting demand for biological safety testing.
- Major Restraint – Cost Constraints: High testing costs pose a significant challenge, particularly for smaller companies with limited resources.
- Opportunity – Outsourcing Trend: Growing outsourcing of biological safety testing services provides opportunities for service providers to meet rising demand.
- Technological Advancements: Advancements in testing methods, including 3D tissue models, are contributing to market growth.
- Regional Dominance: North America led with over 36.2% market share in 2023, driven by advanced healthcare infrastructure and strict regulations.
Get Sample PDF Report: https://market.us/report/biological-safety-testing-market/request-sample/
Biological Safety Testing Market Key Segments
Product
- Reagents & Kits
- Instruments
- Services
Application
- Vaccines & Therapeutics
- Vaccines
- Monoclonal Antibodies
- Recombinant Protein
- Blood & Blood-based Products
- Gene Therapy
- Tissue & Tissue-based Products
- Stem Cell
Test Type
- Endotoxin Tests
- Sterility Tests
- Cell Line Authentication & Characterization Tests
- Bioburden Tests
- Adventitious Agent Detection Tests
- Residual Host Contamination Detection Tests
- Others
Key Regions
- North America (The US, Canada, Mexico)
- Western Europe (Germany, France, The UK, Spain, Italy, Portugal, Ireland, Austria, Switzerland, Benelux, Nordic, Rest of Western Europe)
- Eastern Europe (Russia, Poland, The Czech Republic, Greece, Rest of Eastern Europe)
- APAC (China, Japan, South Korea, India, Australia & New Zealand, Indonesia, Malaysia, Philippines, Singapore, Thailand, Vietnam, Rest of APAC)
- Latin America (Brazil, Colombia, Chile, Argentina, Costa Rica, Rest of Latin America)
- Middle East & Africa (Algeria, Egypt, Israel, Kuwait, Nigeria, Saudi Arabia, South Africa, Turkey, United Arab Emirates, Rest of MEA)
Buy Directly: https://market.us/purchase-report/?report_id=39656
Key Players Analysis
Charles River Laboratories offers comprehensive biological safety testing services, focusing on the safety and efficacy of biologics. Their offerings include viral clearance, cell line characterization, and endotoxin testing. The company leverages advanced technologies and a global network of facilities to ensure compliance with regulatory standards, supporting pharmaceutical and biotechnology firms in bringing safe products to market efficiently.
BSL Bioservice provides a wide range of biological safety testing services tailored to the pharmaceutical and medical device industries. Their expertise includes biocompatibility testing, microbiological testing, and toxicology studies. They specialize in both in vivo and in vitro bioassays, ensuring products meet stringent safety and efficacy standards. The company’s comprehensive testing strategies support clients from early development through to final product release.
Merck KGaA, operating as MilliporeSigma in the U.S. and Canada, excels in biological safety testing through its BioReliance® services. The company offers robust testing solutions for viral clearance, genetic stability, and contamination control. MilliporeSigma supports the entire product lifecycle, from development to commercialization, ensuring biopharmaceutical products meet global regulatory requirements.
Samsung Biologics provides extensive biological safety testing services as part of its integrated biopharmaceutical manufacturing and development solutions. Their testing capabilities include viral safety, sterility testing, and cell line characterization. Samsung Biologics ensures high standards of quality and compliance, facilitating the development of safe and effective biologics.
Sartorius AG offers a range of biological safety testing solutions focused on the biopharmaceutical industry. Their services encompass mycoplasma testing, sterility testing, and virus safety. With state-of-the-art technologies and global regulatory expertise, Sartorius AG helps clients ensure the safety and quality of their biopharmaceutical products.
Biological Safety Testing Market Key Players:
- Charles River Laboratories
- BSL Bioservice
- Merck KGaA (MilliporeSigma)
- Samsung Biologics
- Sartorius AG
- Eurofins Scientific
- SGS Société Générale de Surveillance SA
- Thermo Fisher Scientific Inc.
- BIOMÉRIEUX
- Lonza
Biological Safety Testing Market Report Scope >> Market Value (2023): USD 4.8 Billion || Forecast Revenue (2033): USD 13.4 Billion || CAGR (2024-2033): 10.9% || Base Year Estimation: 2023 || Historic Period: 2019-2022 || Forecast Period: 2024-2033.
Inquire More about report: https://market.us/report/biological-safety-testing-market/#inquiry
About Market.US
Market.US is renowned for its comprehensive market research and analysis, providing customized and syndicated reports to a global clientele. Specializing in a variety of sectors, they offer strategic insights and detailed market forecasts, assisting businesses in making informed decisions. With a focus on innovation and accuracy, Market.US supports clients in over 126 countries, and maintains a strong repeat customer rate, underscoring their commitment to quality and client satisfaction. Their team excels in delivering exceptional research services, ensuring that no detail is overlooked in any target market.
Contact Details
Market.us (Powered By Prudour Pvt. Ltd.)
Contact No: +1 718 618 4351.
Email: inquiry@market.us
Blog: https://medicalmarketreport.com/
View More Trending Reports
Muscle Stimulator Market Outlook: Expected To Expand To USD 1158.7 Million Upholding A 4.3% CAGR
Tinnitus Management Industry Set for Impressive Growth, Projected to Hit USD 4 Billion By 2033
Phoropters Market Expected to Reach New Heights, Reaching USD 160.5 Million by 2033
Editor Details
-
Company:
- Wired Release
- Website:
