
Global Persistent Epithelial Defect Management Market Set to Surge to USD 44.49 Billion by 2033 at a 18% of CAGR
 Global Persistent Epithelial Defect Management Industry
Global Persistent Epithelial Defect Management Industry
The global persistent epithelial defect management market is projected to reach a valuation of USD 8.5 billion in 2023, with significant growth anticipated over the next decade. According to Future Market Insights, the market is expected to expand at an impressive compound annual growth rate (CAGR) of 18%, reaching approximately USD 44.49 billion by 2033.
This remarkable growth is attributed to the rising prevalence of ocular surface disorders, increasing awareness about innovative treatment methodologies, and advancements in medical technologies. As healthcare providers focus on improving patient outcomes and quality of life, the demand for effective management solutions for persistent epithelial defects is expected to escalate.
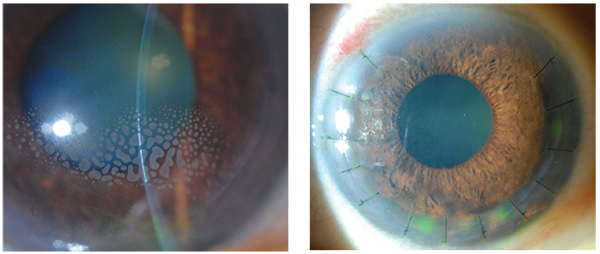
Persistent epithelial defects (PEDs) result from the failure of rapid re-epithelialization and closure within 10-14 days after a corneal injury, even with standard supportive treatment. In recent years, PED awareness has increased with increased research activities from various spectrums of ophthalmology research. However, the actual frequency of PED is not known as of now, but some studies have estimated the frequency based on the causative occurrence of the disease with underlying conditions.
Some of the symptoms of PEDs are loss of corneal epithelial cells, delayed epithelialization post-injury, nonhealing epithelial defect, and others which can be commonly due to Epithelial/limbal, inflammatory disease, Neurotrophic, mechanical causes, and others.
The multi-layered corneal epithelium acts as a protective barrier to infectious agents via tight junctions between neighboring cells, and it maintains its smooth optical surface by constantly regenerating cells in the basal cell layer. Disruptions in this protective layer can render the eye susceptible to infection, stromal ulceration, perforation, scarring, and decreased visual acuity.
It covers the details of conventional and current medical therapies and diagnoses available in the persistent epithelial defect (PED) market to treat the condition. It also provides country-wise treatment guidelines and algorithms across the United States, Europe, and Japan.
Prominent Growth Drivers Influencing Persistent Epithelial Defect Management Market
Increased Partnerships and Collaborations
The increasing partnerships and collaborations between numerous companies boost market growth. For instance, in October 2017, Bio-Tissue entered a strategic agreement with Optima Pharmazeutische GmbH, a pharmaceutical company that offers otorhinolaryngology, ophthalmology, and pneumology products. This agreement helps the company to distribute its Natural Ocular Cleanser in Germany. Thus, all these collaborations boost the growth of the market.
Higher Demand for Hospitals
The increasing demand for hospitals surges the market grows rapidly. Most chronic disease diagnostics are majorly performed in hospitals since they are very complex and it requires technologically advanced products and thus this is boosting the market for hospital/clinical laboratories.
How Competitive is the Market for Persistent Epithelial Defect Management Treatment?
The persistent epithelial defects management treatment market competitive landscape provides details of competitors. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width, and breadth, and application dominance. The above data points provided are only related to the companies’ focus related to the persistent corneal epithelial defects treatment market.
Some of the important developments of the key players in the market are
- Kala Pharmaceuticals acquired Combangio, expanding its pipeline with a clinical-stage novel biologic for the treatment of Persistent Epithelial Defect (PED) and other rare ocular surface diseases.
- Nexagon has a market advantage in the US, as it received orphan drug designation from the FDA. It is being developed by OcuNexus Therapeutics in partnership with Eyevance Pharmaceuticals. Currently, the drug is under phase II studies for PED.
Key players operating in the persistent epithelial defects treatment market t include
- Dompe Farmaceutici
- OcuNexus Therapeutics
- Eyevance Pharmaceuticals
- Noveome Biotherapeutics
- RegeneRx Biopharmaceuticals
- Recordati Rare Diseases
- Mimetech
- Johnson & Johnson Services Inc.
- Bausch Health Companies Inc.
- Integra Lifesciences
Key Segments Profiled in the Persistent Epithelial Defect Management Market Report
Disease Type:
- Epithelial/Limbal
- Inflammatory Disease
- Neurotrophic Disease
- Others
End User:
- Hospitals/Clinical Laboratories
- Physician Offices
- Reference Laboratories
- Other End Users
Region:
- North America
- Latin America
- Europe
- East Asia
- South Asia
- Oceania
- Middle East & Africa
Author By:
Sabyasachi Ghosh (Associate Vice President at Future Market Insights, Inc.) holds over 12 years of experience in the Healthcare, Medical Devices, and Pharmaceutical industries. His curious and analytical nature helped him shape his career as a researcher.
Identifying key challenges faced by clients and devising robust, hypothesis-based solutions to empower them with strategic decision-making capabilities come naturally to him. His primary expertise lies in areas such as Market Entry and Expansion Strategy, Feasibility Studies, Competitive Intelligence, and Strategic Transformation.
Holding a degree in Microbiology, Sabyasachi has authored numerous publications and has been cited in journals, including The Journal of mHealth, ITN Online, and Spinal Surgery News.
About Future Market Insights (FMI)
Future Market Insights, Inc. (ESOMAR certified, recipient of the Stevie Award, and a member of the Greater New York Chamber of Commerce) offers profound insights into the driving factors that are boosting demand in the market. FMI stands as the leading global provider of market intelligence, advisory services, consulting, and events for the Packaging, Food and Beverage, Consumer Technology, Healthcare, Industrial, and Chemicals markets. With a vast team of over 400 analysts worldwide, FMI provides global, regional, and local expertise on diverse domains and industry trends across more than 110 countries.
Contact Us:
Future Market Insights Inc.
Christiana Corporate, 200 Continental Drive,
Suite 401, Newark, Delaware – 19713, USA
T: +1-347-918-3531
For Sales Enquiries: sales@futuremarketinsights.com
Website: https://www.futuremarketinsights.com
LinkedIn| Twitter| Blogs | YouTube
Editor Details
-
Company:
- MARKITWIRED
- Website:
