
Global Companion Diagnostics Market to Surpass USD 11,682.8 Million by 2035 amid Rising Demand for Personalized Medicine and Technological Advancements | FMI
 Companion Diagnostics Market
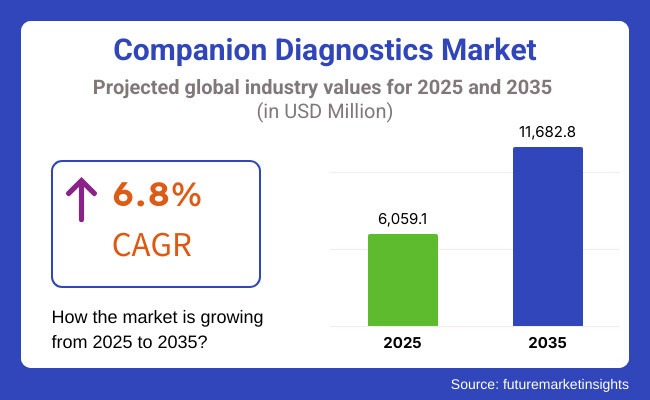
Companion Diagnostics Market
The global market for companion diagnostics is set for exponential growth, projected to expand from USD 5,700.5 million in 2024 to USD 11,682.8 million by 2035, registering a compound annual growth rate (CAGR) of 6.8% during the forecast period (2025–2035). With revenue expected to reach USD 6,059.1 million by 2025, the CDx industry continues to demonstrate its pivotal role in shaping the future of precision medicine.
The surge in the companion diagnostics market is attributed to an increased demand for personalized medicine and the rapid pace of innovation in targeted drug development. Pharmaceutical companies are heavily investing in customized therapeutics, and CDx is playing an indispensable role in identifying the most effective treatment for individual patients, particularly in oncology.
Explore Key Trends in the Market: Request Your Sample Report! https://www.futuremarketinsights.com/report-sample#5245502d47422d31323535
Personalized Medicine Leading the Charge
The growing preference for individualized treatment pathways is propelling the widespread adoption of companion diagnostics. These tests are essential in identifying patient subgroups likely to benefit from specific therapies, particularly in cancers and chronic diseases. The ability to tailor treatments based on molecular and genetic profiles significantly enhances clinical outcomes while optimizing healthcare resources.
Regulatory support from agencies like the U.S. FDA and the European Medicines Agency (EMA) has further validated the use of CDx, requiring them in tandem with new drug approvals. This regulatory push has led to an accelerated adoption rate across global markets.
Technological Advancements Driving Market Expansion
Next-generation sequencing (NGS), liquid biopsy, and AI-powered diagnostic tools are ushering in a new era of precision diagnostics. These technologies not only improve the speed and accuracy of testing but also reduce costs, enabling broader applications of CDx across diverse therapeutic areas. With continued R&D, companion diagnostics are expected to expand beyond oncology into neurology, infectious diseases, and even rare genetic disorders.
Additionally, collaborations between pharmaceutical and diagnostic companies are enabling the co-development of innovative multi-gene panels. These panels, powered by advanced bioinformatics and big data, allow comprehensive analysis for more precise treatment recommendations.
Future Outlook: The Era of Theranostics and Digital Pathology
The future of the CDx market is bright, driven by innovations in digital pathology, artificial intelligence, and theranostics—a field combining diagnostics and therapeutics. Multi-gene panels and real-time monitoring of disease progression are expected to dominate the landscape, offering more dynamic and responsive treatment options. As these technologies mature, the role of CDx in the management of chronic and complex diseases will only deepen.
Market Evolution and Historical Context
The evolution of companion diagnostics has mirrored the broader shift toward precision medicine. Initially centered around biomarker-based testing in oncology, CDx has now become a cornerstone of personalized healthcare. Regulatory guidance, particularly in the U.S. and Europe, has accelerated the integration of diagnostics with therapeutic decisions.
Technological improvements in NGS and non-invasive testing methods such as liquid biopsy have increased the accessibility and utility of CDx, allowing clinicians to gain real-time insights into disease behavior and progression. As the market matures, newer clinical applications in neurology and infectious disease management are emerging.
Increased Market Demand: Get In-Depth Analysis and Insights with Our Complete Report! https://www.futuremarketinsights.com/reports/companion-diagnostics-market
Key Regional Insights: Global Expansion in Focus
- United States: The U.S. companion diagnostics market continues to lead globally, buoyed by strong healthcare infrastructure, high R&D investments, and widespread adoption of precision therapies.
CAGR (2025–2035): 6.0%
- Germany: Germany’s robust healthcare framework and continuous research in genomics and biomarker discovery support a steady market climb.
CAGR (2025–2035): 6.5%
- China: A rising focus on healthcare modernization, precision medicine, and biotech investment positions China for aggressive growth.
CAGR (2025–2035): 7.7%
- India: India’s increasing disease awareness, public-private partnerships, and infrastructure improvements are fueling CDx adoption.
CAGR (2025–2035): 8.1%
- Brazil: The market in Brazil is growing, supported by government initiatives and rising demand for personalized healthcare solutions.
CAGR (2025–2035): 7.0%
Competitive Landscape: A Dynamic Ecosystem
The companion diagnostics market is characterized by intense competition, continual innovation, and strong collaboration between biotech firms, diagnostic players, and pharmaceutical companies. Key industry players are actively investing in emerging technologies like AI-powered diagnostics, gene sequencing platforms, and advanced liquid biopsy methods to maintain their competitive edge.
Leading Companies:
- F. Hoffmann-La Roche AG
- Qiagen Ltd.
- bioMérieux Inc.
- Abbott
- Thermo Fisher Scientific Inc.
- Myriad Genetics Inc.
- Dako Inc.
- Biogenex Laboratories, Inc.
- ARUP Laboratories
- Ventana Medical Systems Inc.
- Leica Biosystems Nussloch GmbH
These firms are spearheading initiatives to develop new biomarkers and co-develop tests alongside novel therapeutics, ensuring more comprehensive and effective treatment solutions.
Key Market Segmentation
By Product:
Assay, kits & reagents and software and services
By Technology:
Immunohistochemistry, molecular diagnostics, in-situ hybridization, real time PCR, and gene sequencing
By Application:
Colorectal cancer, breast cancer, lung cancer, melanoma, urology, and gastric cancer
By End User:
Pharma and biotech companies, clinical research organizations, reference laboratories and others
By Region:
North America, Latin America, Western Europe, Eastern Europe, East Asia, South Asia & Pacific, Middle East & Africa
Download the Complete Healthcare Sector Report for Decision-Makers! https://www.futuremarketinsights.com/industry-analysis/life-science-and-biotechnology
Conclusion
The companion diagnostics market is undergoing a significant transformation, powered by the rising demand for precision medicine, technological breakthroughs, and an evolving regulatory landscape. As CDx becomes increasingly essential in guiding therapeutic choices, the market is primed for sustainable and inclusive growth across regions and clinical specialties.
With a clear trajectory toward broader applications and deeper integration into healthcare systems, companion diagnostics are poised to redefine how the world approaches disease treatment and management over the next decade.
Editor Details
-
Company:
- MARKITWIRED
- Website:
